理想気体法律
概要
ソース: 博士アンドレアス Züttel - スイス連邦研究所材料工学講座
理想気体法律は近く周囲条件下で最も一般的なガスの挙動と希薄限界にすべての化学物質の傾向について説明します。それは 3 つの測定可能なマクロスコ ピック システム変数 (圧力、温度と体積) とガスの分子の数との間の基本的関係システムで、したがって、微視的および巨視的な宇宙間の重要なリンク。
理想気体法律の歴史 17th世紀圧力と空気量の関係が反比例することが判明したときロバート ・ ボイルと我々 はすぐに参照してボイルの法則 (式 1) で確認した式の中間に日付を記入します。
P 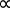 V-1 (式 1)
V-1 (式 1)
ジョセフ ・ ルイ ・ ゲイ = リュサックによって多数のガスや蒸気に拡張され、1802 年に報告された、1780 年代にジャック ・ シャルルによる未発行作業は、絶対温度と気体の体積の比例関係を設立しました。この関係は、シャルルの法則 (式 2) と呼ばれます。
V 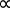 T (式 2)
T (式 2)
ギヨーム ・ アモントンは18 世紀の変わり目に固定ボリューム内の空気の圧力と温度の関係を発見した通常入金されます。この法律19 世紀の初めのジョセフ ・ ルイ ・ ゲイ = リュサックによって他の多くのガスにも拡張された、アモントンの法則やゲイ ・ リュサックの法則、式 3のように記載されているといいます。
P 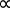 T (式 3)
T (式 3)
一緒に、これらの 3 つの関係は式 4の関係を与えるため結合できます。
V 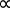 T (式 4)
T (式 4)
最後に、1811 年、それにより提案されましたアメデオ ・ アボガドロの分子の同じ数を含む任意の 2 つのガスは、同じボリューム、同じ温度および圧力を開催しました。これはすべてのガスを記述できます一般的な定数、理想気体定数 R は気体の性質から独立した結論に導いた。これは理想気体法律 (式 5) として知られています。1, 2
太陽光発電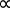 T (式 5)
T (式 5)
手順
1. サンプルの体積を測定
- サンプルを慎重に洗浄及び乾燥しています。
- 十分な高解像度のメスシリンダーを記入サンプルをカバーする蒸留水します。最初のボリュームに注意してください。
- 水の中にサンプルを削除し、ボリュームの変更に注意してください。これは、サンプルでは、V の量です。
- サンプルを削除して、それを乾燥します。注: また、測定サンプルの側 length(s) とジオメトリを使用してそのボリュームを計算します。
2. 負荷バランスのサンプル
- 磁気浮上天秤でサンプルをハングします。
- サンプルのまわりの圧力/温度チャンバーをインストールします。
- サンプル環境を避難させると 1 つのバーに水素ガスを補充します。
- 1 のバーと室内の温度、w では試料重量を測定00 。
3 常温で圧力の関数としての測定試料重量
結果
申請書と概要
参考文献
- Zumdahl, S.S., Chemical Principles. Houghton Mifflin, New York, NY. (2002).
- Kotz, J., Treichel, P., Townsend, J. Chemistry and Chemical Reactivity. 8th ed. Brooks/Cole, Belmont, CA (2012).
- Rouquerol, F., Rouquerol, J., Sing, K.S.W., Llewellyn, P., Maurin, G. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications.Academic Press, San Diego, CA. (2014).
タグ
スキップ先...
このコレクションのビデオ:

Now Playing
理想気体法律
General Chemistry
78.5K 閲覧数

共通の実験室ガラス製品と用途
General Chemistry
656.1K 閲覧数

・濃度
General Chemistry
274.2K 閲覧数

固体と液体の密度を決定します。
General Chemistry
556.1K 閲覧数

水溶液の質量パーセントの組成を決定します。
General Chemistry
383.5K 閲覧数

経験式を決定します。
General Chemistry
181.9K 閲覧数

イオン性化合物の溶解度ルールの決定
General Chemistry
141.4K 閲覧数

PH メーターを使用してください。
General Chemistry
345.9K 閲覧数

滴定の概要
General Chemistry
424.5K 閲覧数

平衡定数の吸光光度定量
General Chemistry
158.5K 閲覧数

ル Châtelier の原理
General Chemistry
265.2K 閲覧数

未知の化合物を決定するための凝固点降下
General Chemistry
160.7K 閲覧数

率の法律および反作用の順序を決定します。
General Chemistry
196.1K 閲覧数

エンタルピーの示差走査熱量測定の変更を使用してください。
General Chemistry
44.5K 閲覧数

錯体化学
General Chemistry
91.5K 閲覧数
Copyright © 2023 MyJoVE Corporation. All rights reserved
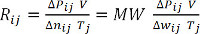 (式 9)
(式 9)