Calibration Curves
Source: Laboratory of Dr. B. Jill Venton - University of Virginia
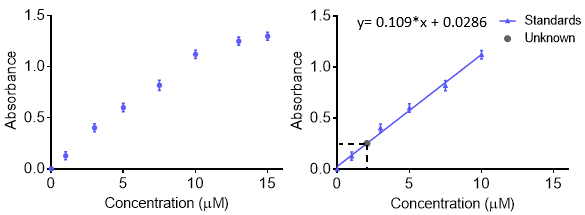
Calibration curves are used to understand the instrumental response to an analyte and predict the concentration in an unknown sample. Generally, a set of standard samples are made at various concentrations with a range than includes the unknown of interest and the instrumental response at each concentration is recorded. For more accuracy and to understand the error, the response at each concentration can be repeated so an error bar is obtained. The data are then fit with a function so that unknown concentrations can be predicted. Typically the response is linear, however, a curve can be made with other functions as long as the function is known. The calibration curve can be used to calculate the limit of detection and limit of quantitation.
When making solutions for a calibration curve, each solution can be made separately. However, that can take a lot of starting material and be time consuming. Another method for making many different concentrations of a solution is to use serial dilutions. With serial dilutions, a concentrated sample is diluted down in a stepwise manner to make lower concentrations. The next sample is made from the previous dilution, and the dilution factor is often kept constant. The advantage is that only one initial solution is needed. The disadvantage is that any errors in solution making—pipetting, massing, etc.—get propagated as more solutions are made. Thus, care must be taken when making the initial solution.
1. Making the Standards: Serial Dilutions
- Make a concentrated stock solution of the standard. Typically, the compound is accurately weighed out and then quantitatively transferred into a volumetric flask. Add some solvent, mix so the sample dissolves, then fill to the line with the proper solvent.
- Perform serial dilutions. Take another volumetric flask and pipette the amount of standard needed for the dilution, then fill to the line with solvent and mix. A ten-fold dilution is typically made, so for
Calibration curves are used in many fields of analytical chemistry, biochemistry, and pharmaceutical chemistry. It is common to use them with spectroscopy, chromatography, and electrochemistry measurements. A calibration curve can be used to understand the concentration of an environmental pollutant in a soil sample. It could be used determine the concentration of a neurotransmitter in a sample of brain fluid, vitamin in pharmaceutical samples, or caffeine in food. Thus, calibration curves are useful in environmental, bi
건너뛰기...
이 컬렉션의 비디오:

Now Playing
Calibration Curves
Analytical Chemistry
789.9K Views

분석 특성화를 위한 샘플 준비
Analytical Chemistry
83.8K Views

내부 표준
Analytical Chemistry
203.9K Views

표준 추가 방법
Analytical Chemistry
318.9K Views

자외선 눈에 보이는 (UV-Vis) 분광법
Analytical Chemistry
617.4K Views

화학 분석을 위한 라만 분광법
Analytical Chemistry
50.9K Views

엑스레이 형광 (XRF)
Analytical Chemistry
25.3K Views

화염 이온화 감지를 갖춘 가스 크로마토그래피(GC)
Analytical Chemistry
280.1K Views

고성능 액체 크로마토그래피 (HPLC)
Analytical Chemistry
382.1K Views

이온 교환 크로마토그래피
Analytical Chemistry
263.1K Views

모세관 전기 포레시스 (CE)
Analytical Chemistry
93.1K Views

질량 분광법 소개
Analytical Chemistry
111.5K Views

스캐닝 전자 현미경 검사법 (SEM)
Analytical Chemistry
86.6K Views

전위요스타트/갈바노스타트를 사용하여 지원되는 촉매의 전기화학적 측정
Analytical Chemistry
51.2K Views

순환 볼탐량 (CV)
Analytical Chemistry
123.6K Views
Copyright © 2025 MyJoVE Corporation. 판권 소유