Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Lipid Vesicle-mediated Affinity Chromatography using Magnetic Activated Cell Sorting (LIMACS): a Novel Method to Analyze Protein-lipid Interaction
W tym Artykule
Podsumowanie
To test the interaction of a protein with its target lipid we used MACS and Annexin V-conjugated magnetic beads and lipid vesicles synthesized from the target lipid and Annexin V-binding phosphatidylserine. Proteins bound to the target lipid are co-purified and analyzed after elution from the beads.
Streszczenie
The analysis of lipid protein interaction is difficult because lipids are embedded in cell membranes and therefore, inaccessible to most purification procedures. As an alternative, lipids can be coated on flat surfaces as used for lipid ELISA and Plasmon resonance spectroscopy. However, surface coating lipids do not form microdomain structures, which may be important for the lipid binding properties. Further, these methods do not allow for the purification of larger amounts of proteins binding to their target lipids.
To overcome these limitations of testing lipid protein interaction and to purify lipid binding proteins we developed a novel method termed lipid vesicle-mediated affinity chromatography using magnetic-activated cell sorting (LIMACS). In this method, lipid vesicles are prepared with the target lipid and phosphatidylserine as the anchor lipid for Annexin V MACS. Phosphatidylserine is a ubiquitous cell membrane phospholipid that shows high affinity to the protein Annexin V. Using magnetic beads conjugated to Annexin V the phosphatidylserine-containing lipid vesicles will bind to the magnetic beads. When the lipid vesicles are incubated with a cell lysate the protein binding to the target lipid will also be bound to the beads and can be co-purified using MACS. This method can also be used to test if recombinant proteins reconstitute a protein complex binding to the target lipid.
We have used this method to show the interaction of atypical PKC (aPKC) with the sphingolipid ceramide and to co-purify prostate apoptosis response 4 (PAR-4), a protein binding to ceramide-associated aPKC. We have also used this method for the reconstitution of a ceramide-associated complex of recombinant aPKC with the cell polarity-related proteins Par6 and Cdc42. Since lipid vesicles can be prepared with a variety of sphingo- or phospholipids, LIMACS offers a versatile test for lipid-protein interaction in a lipid environment that resembles closely that of the cell membrane. Additional lipid protein complexes can be identified using proteomics analysis of lipid binding protein co-purified with the lipid vesicles.
Protokół
1. Introduction
The lipid vesicle-mediated affinity chromatography using magnetic-activated cell sorting (LIMACS) technique was developed in our laboratory to isolate ceramide-associated protein complexes 1-3. Originally, the lipid vesicles were made of ceramide and phosphatidylserine, which allowed for MACS using magnetic particle-conjugated Annexin V (highly affine to phosphatidylserine) to isolate the vesicles and their associated proteins. We have used the LIMACS technique for the in vitro reconstitution of a ceramide-associated polarity complex and the isolation of ceramide-binding proteins from cell lysates 3. LIMACS can be modified using other interaction partners for the isolation of the vesicles (e.g., glycolipid-specifc lectins or lipid antibodies).
2. Experimental Procedures
Preparation of Lipid Vesicles and aPKCBinding Assays
- Lipid vesicles are obtained from dried mixtures of equimolar amounts of phosphatidylserine (105 μg) and C16-ceramide (85 μg) following modified procedures for large liposome preparation 1,4-7.
- The lipid mixtures are then resuspended and sonicated for 1 h in 100 μl of vesicle buffer consisting of 50 mM Tris/HCl (pH 7.5) and 150 mM NaCl.
- After adding 300 μl of vesicle buffer supplemented with 0.1 mM MnCl2, the samples are centrifuged at 12,000 μg for 20 min at 4 °C.
- The pellet (large lipid vesicles) is resuspended in 100 μl of vesicle buffer and incubated with 1 nmol of Vybrant CM-diI for 1 h at 37 °C to visualize the vesicle fraction after MACS separation. Vybrant CM-diI is a red fluorescent dye specifically incorporating into lipid membranes.
- A detergent-free cell lysate is prepared by sonication/homogenization of cells in 300 μl of hypotonic buffer (10 mM Tris/HCl (pH 7.0) with protease and phosphatase inhibitors) followed by removal of membranous debris by centrifugation. A centrifugation step at 100,000xg for 1 h should be added to avoid contamination of the cell lysate with endogenous membranes containing phosphatidylserine.
- The cleared lysate is added to the lipid vesicle suspension, and the mixture is incubated for 2 h at 4 °C.
- The reaction mixture is supplemented with 20 μl of 20x Annexin V binding buffer and 50 μl of a solution containing magnetic beads conjugated to Annexin V followed by incubation for 1 h at 4 °C.
- MACS is performed according to the manufacturer's (Miltenyi Biotec, Inc.) protocol. The presence and quantity of lipid vesicles is determined by monitoring the Vybrant CM-diI fluorescence in the flow-through and elution fractions using a microplate fluorescence reader.
- The linear correlation between the amount of vesicular lipid and fluorescence intensity of vesicle-bound Vybrant CM-diI is verified by quantitative high-performance thin-layer chromatography (HPTLC) of the lipid mixture applied to Annexin V-based MACS.
- The specificity of the binding reaction of aPKC or other proteins to the ceramide/phosphatidylserine vesicles is verified by an antibody competition assay using 1 μg of anti-PKCζ rabbit polyclonal antibody to incubate the cell lysate for 1 h at 4 °C prior to incubation with the lipid vesicles.
- The protein binding to ceramide/phosphatidylserine vesicles in the MACS eluate is analyzed by SDS-PAGE and immunoblotting.
In vitro lipid-protein polarity complex
- The in vitro reconstitution of a lipid-protein polarity complex is performed following the LIMACS procedure as described in the previous section. In brief, phosphatidylserine (420 μg) and C16-ceramide (107 μg) is dried from organic solvent.
- The dried lipids are resuspended under sonication in 500 μl of vesicle buffer (50 mM Tris/HCl, pH 7.5; 150 mM NaCl).
- Five μl of 10 mM MnCl2, 1 μl of Vybrant CM-diI, and 500 ng of PKCζ (human recombinant) is added and the reaction mixture incubated undergentle agitation for 60 min at 4 °C.
- Vybrant CM-diI stained phosphatidylserine/ceramide vesicles are recovered by centrifugation at 12,000xg for 60 min at 4 °C.
- The pellet (pink) is resuspended in 100 μl Tris buffer and supplemented with γS-GTP (100 μM), GDP (1 mM), GST-Par6 (100 ng), or GST-Cdc42 (500 ng) and further incubated for 3 h at 4 °C (any other combination of recombinant proteins of interest can be used here).
- Annexin V-buffer (5 μl of a 20x stock solution) and Annexin V-conjugated magnetic beads (50 μl) are added and the reaction mixture incubated under gentle agitation for another 30 min at 4 °C.
- Annexin V-MACS is performed following the supplier's protocol as described previously.
- The elution fraction (1 ml) is supplemented with 10 μg of pure ovalbumin as precipitation aid. The protein is concentrated by Wessel-Flugge precipitation and analyzed by SDS-PAGE/immunoblotting as previously described 8.
- The amount of eluted lipid vesicles is quantified by the detection of Vybrant CM-diI (pink) in the organic (chloroform/methanol) phase of the Wessel Flugge precipitation reaction. The amount of protein analyzed is normalized on equal amounts of lipid vesicles.
3. Results
LIMACS purification of PKCζ-EGFP and the ceramide binding domain C20ζ-EGFP
A detergent-free lysate of MDCK cells expressing full length PKCζ C-terminally linked to green fluorescent protein (FLζ-EGFP) or a ceramide binding domain in the C-terminus of PKCζ (C20ζ-EGFP) was incubated with phosphatidylserine/ceramide vesicles as described in Experimental Procedures. After elution of the MACS column, protein was analyzed using immunoblotting and antibodies against PKCζ and EGFP for detection of the eluted protein 2.
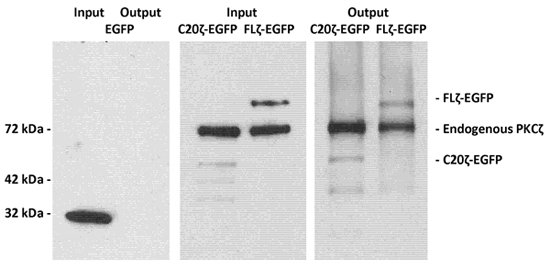
Figure 1. LIMACS of EGFP-labeled PKCζ and its C-terminal fragment C20ζ using phosphatidylserine/ceramide vesicles.
Detergent-free lysates of MDCK cells expressing EGFP (as a non-binding control), full length PKCζ-EGFP, or the ceramide binding, C-terminal fragment C20ζ-EGFP were incubated with phosphatidylserine/ceramide vesicles as described in the Experimental Procedures section. After using LIMACS, protein was eluted with SDS sample buffer and analyzed by SDS-PAGE and immunoblotting. The left panel shows that EGFP did not bind to the vesicles retained with the Annexin V-linked magnetic beads. The middle and right panel shows that full length PKCζ-EGFP and C20ζ-EGFP were retained due to binding to ceramide.
Dyskusje
To test the specific interaction between a lipid and its binding protein is hampered by embedding of lipids in the cell membrane. The cell membrane consists of a mixture of several lipids and proteins and it is organized in lipid microdomains or rafts. Therefore, the co-purification of microdomains and proteins cannot clearly distinguish if a protein directly binds to a lipid or is only enriched in a microdomain structure. Other methods using defined lipids coated on surfaces such as lipid ELISAs or Plasmon resonance spe...
Ujawnienia
No conflicts of interest declared.
Podziękowania
This work was supported by the NIH grants R01NS046835 and R01AG034389, and the March of Dimes grant 6FY08-322. A special thank you is devoted to Mrs. Eleanor Brown (Miltenyi Biotec, Auburn, CA) who helped tremendously with her insight into the MACS technology. Miltenyi has generously provided the material used for the demonstration of the experiments at no cost. I am also grateful to Dr. Guanghu Wang (Medical College of Georgia/Georgia Health Sciences University, Augusta, GA) who generated the PKCζ expressing cell lines. Support by the Institute of Molecular Medicine at the Medical College of Georgia/Georgia Health Sciences University (under directorship of Dr. Lin Mei) is also acknowledged.
Materiały
| Name | Company | Catalog Number | Comments |
| Annexin V-conjugated magnetic beads and MACS micro and minicolumns | Miltenyi Biotec | ||
| Lipids (of highest purity) | Avanti Polar Lipid, Inc |
Odniesienia
- Wang, G. Direct binding to ceramide activates protein kinase Czeta before the formation of a pro-apoptotic complex with PAR-4 in differentiating stem cells. J Biol Chem. 280, 26415-26424 (2005).
- Wang, G., Krishnamurthy, K., Umapathy, N. S., Verin, A. D., Bieberich, E. The carboxyl-terminal domain of atypical protein kinase Czeta binds to ceramide and regulates junction formation in epithelial cells. J Biol Chem. 284, 14469-14475 (2008).
- Chalfant, C. E. De novo ceramide regulates the alternative splicing of caspase 9 and Bcl-x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase-1. J Biol Chem. 277, 12587-12595 (2002).
- Simon, C. G., Holloway, P. W., Gear, A. R. Exchange of C(16)-ceramide between phospholipid vesicles. Biochemistry. 38, 14676-14682 (1999).
- Goni, F. M., Contreras, F. X., Montes, L. R., Sot, J., Alonso, A. Biophysics (and sociology) of ceramides. Biochem Soc Symp. , 177-188 (2005).
- Kumagai, K. CERT mediates intermembrane transfer of various molecular species of ceramides. J Biol Chem. 280, 6488-6495 (2005).
- Wessel, D., Flugge, U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 138, 141-143 (1984).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone