Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Preparation of Intact Bovine Tail Intervertebral Discs for Organ Culture
W tym Artykule
Podsumowanie
This protocol illustrates a harvesting technique for coccygeal bovine intervertebral discs for organ culture for in vitro organ culture.
Streszczenie
The intervertebral disc (IVD) is the joint of the spine connecting vertebra to vertebra. It functions to transmit loading of the spine and give flexibility to the spine. It composes of three compartments: the innermost nucleus pulposus (NP) encompassing by the annulus fibrosus (AF), and two cartilaginous endplates connecting the NP and AF to the vertebral body on both sides. Discogenic pain possibly caused by degenerative intervertebral disc disease (DDD) and disc herniations has been identified as a major problem in our modern society. To study possible mechanisms of IVD degeneration, in vitro organ culture systems with live disc cells are highly appealing. The in vitro culture of intact bovine coccygeal IVDs has advanced to a relevant model system, which allows the study of mechano-biological aspects in a well-controlled physiological and mechanical environment. Bovine tail IVDs can be obtained relatively easy in higher numbers and are very similar to the human lumbar IVDs with respect to cell density, cell population and dimensions. However, previous bovine caudal IVD harvesting techniques retaining cartilaginous endplates and bony endplates failed after 1-2 days of culture since the nutrition pathways were obviously blocked by clotted blood. IVDs are the biggest avascular organs, thus, the nutrients to the cells in the NP are solely dependent on diffusion via the capillary buds from the adjacent vertebral body. Presence of bone debris and clotted blood on the endplate surfaces can hinder nutrient diffusion into the center of the disc and compromise cell viability. Our group established a relatively quick protocol to "crack"-out the IVDs from the tail with a low risk for contamination. We are able to permeabilize the freshly-cut bony endplate surfaces by using a surgical jet lavage system, which removes the blood clots and cutting debris and very efficiently reopens the nutrition diffusion pathway to the center of the IVD. The presence of growth plates on both sides of the vertebral bone has to be avoided and to be removed prior to culture. In this video, we outline the crucial steps during preparation and demonstrate the key to a successful organ culture maintaining high cell viability for 14 days under free swelling culture. The culture time could be extended when appropriate mechanical environment can be maintained by using mechanical loading bioreactor. The technique demonstrated here can be extended to other animal species such as porcine, ovine and leporine caudal and lumbar IVD isolation.
Protokół
1. Intervertebral Disc Harvesting
- Whole length bovine tail is obtained from a local abattoir, if possible without skin since the presence of skin increases the chance of contamination (Fig 2).
- Prepare large cutting board and prepare sterile work station and instruments on top of a cutting board (Fig. 2).
- Prepare under the sterile laminar flow hood sterile gauze moistened with 0.9% sodium chloride containing 55mM sodium citrate and put into each well of the 6-well plate.
- Prepare a basin and dilute 1:100 Betadine solution with tap water.
- Immerse the whole bovine tail in a basin containing 1% Betadine solution for 5 min.
- Briefly dry tail with sterile gauze and place it on sterile work station.
- Carefully remove muscle around the tail with scalpel blade #22.
- Chop away the parts of the tail which are not needed, usually both ends of the tail, which contain relatively large and very small IVDs (Fig. 3).
- Trim further the muscle and tendons around the IVD using scalpel blade #10. Beware not to cut the outer annulus of the discs.
- Mark the anterior of the IVD with a surgical skin marker (this step is optional, it helps to determine the center for axial rotation in our bioreactor).
- Locate the IVD and vertebra connection point by moving the tail gently. Feel the border between IVD and bone with the dull side of a scalpel blade. The cleaving site should be 1-2 mm away of the IVD towards the vertebra. Place custom made industrial blade holder on the cleaving site (Fig. 4).
- Cleave IVD and vertebra by hammering on top of the custom-made industrial blade holder (Fig. 3).
- Repeat on the other side of the IVD-vertebra connection.
- Wrap isolated IVD with sterile gauze moistened with 0.9% sodium chloride containing 55mM sodium citrate.
- Continue the isolation of IVDs to the desired sample number (usually around 6 can be harvested with diameter between 10-20 mm).
- Connect the jet lavage system (Zimmer, inc.) with sterile Lactated Ringer's solution, 5L bags are a useful size (alternatively sterile PBS or 0.9% saline solution).
- Hold the IVD with forceps and jet-lavage both sides of the endplate surface using Zimmer Pulsavac jet-lavage system (Fig. 1C). The jet gun should be shot on the endplate surface at an angle between 30 - 60° for about 30s on each side. (Figs. 1 and 7)
- Put the IVDs back to the 6-well plate and wrap with moistened gauze while jet washing another disc sample.
- IVDs are ready for organ culture and down-stream applications (e.g. cell viability, mechanical loading, organ culture) (Fig. 1D).
2. Representative Results
Outcome measurements to judge a successful intervertebral disc preparation can be a diffusion experiment where a small molecular weight fluorescent dye (e.g. procion red) is prepared as a 1% solution in PBS2. The IVD is then kept with at least 100 mL of dye solution and the dye is then allowed to diffuse passively into the IVD for 24h under free swelling. The IVD is then flash-frozen in liquid nitrogen and brought back to room temperature and dehydrated by a series of acetone transfers (first pre-cooled in -80 °C, then -20 °C, then 4 °C and finally to room temperature). The disc can then be embedded in poly-methyl-acrylate (PMMA) and cut with a sharp blade to produce 100 μm-thick sections. These sections can then be viewed by any standard brightfield microscope but preferentially by usage of fluorescence microscopy since procion red emits a red fluorescence (Figs. 5-6). The trabeculi of the bony surface appear very clean after the jet lavaging step (Fig. 7).
Parameters for a successful organ culture are first of all the absence of contamination, maintained cell viability (Figs. 8-9), disc height and overall "disc integrity", measured with histological sections and safranin O/fast green stain or Meyer's Hematoxilin 3. We developed protocols and macros published elsewhere4,5 to stain live disc tissue and to scan cell viability in 3D using a confocal laser scanning microscope and the live/dead staining kit (Molecular Probes, Invitrogen, Basel, Switzerland). We further developed a macro and routine to perform automated cell counting to estimate cell viability in 3D tissue or 3D carriers using the NIH ImageJ platform4-6. Alternate protocols have been proposed to determine cell viability of disc cells by initial digestion using sequential digestion of the extracellular matrix using pronase and collagenase type 2 and then to stain the cell suspension7. An earlier version of the protocol involved an incubation step in PBS containing ten times more concentrated penicillin/streptomycin concentration for 10min after the harvesting of the discs prior organ culture. With the jet lavage step this step becomes obsolete since this step seems beneficial for culturing purposes.

Figure 1. Overview of the methodological steps to prepare bovine coccygeal Intervertebral Discs with intact endplates and a thin layer (1-2mm) of bone. A) Schematic view of the intervertebral disc (IVD) and its nutritional pathways. B) Procedure to cut out the IVD with a customized blade-holder and an industrial sharp blade. C) Jet lavage step to ensure high cell viability and to enable nutrient exchange keeping the endplates and 1-2 mm of bone attached on both sides. D) Finally the IVD can be cultured in the specimen chamber of a bioreactor or be used for any down-stream application.
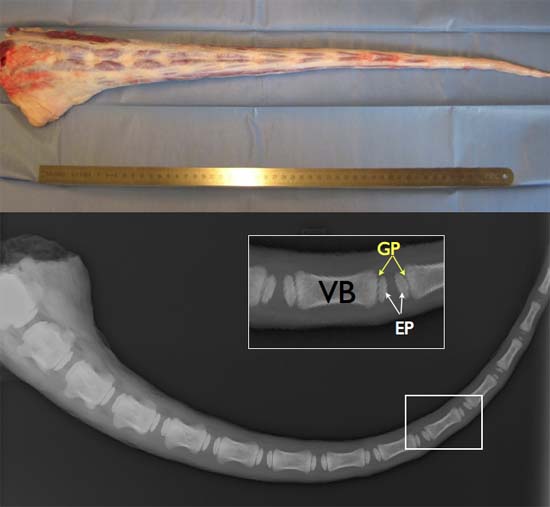
Figure 2. TOP: Full length fresh bovine tail (ideally obtained within 2-3 hours post-mortem to ensure high cell viability. Bottom: X-ray image of a bovine tail aged around 6 months illustrating the existence of growth plates (GP), secondary center of ossification, between the bony endplates (EP) and the vertebra bodies (VB).
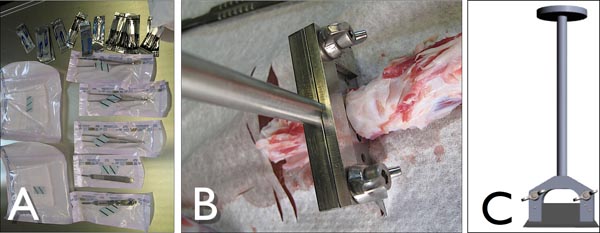
Figure 3. A) Tools to dissect the bovine tail under sterile conditions. B) Customized blade-holder to cut-out motional segments from the bovine tail in action while cutting the intervertebral disc. C) Side-view of the customized blade-holder with a standard industrial blade (Lutz, Germany).

Figure 4. Drawing and picture of the bovine tail after removing the surrounding muscle and ligament illustrating the cleaving sites to cut out the motional segments. Partial vertebra should be "cracked" with the custom-made blade holder ideally 1-2 mm away from the cartilaginous endplates to ensure full bone surface.
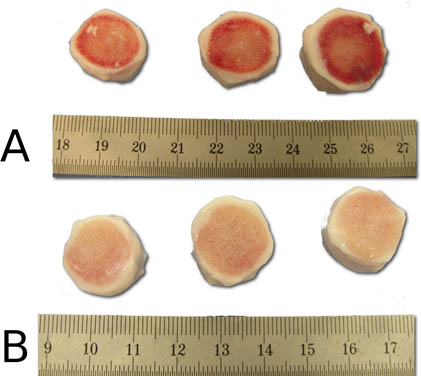
Figure 5. Demonstration of the effective cleaning of the jet-lavage step before (A) and after (B). Notice the clean bony surfaces of the intervertebral disc segments after the spraying procedure.
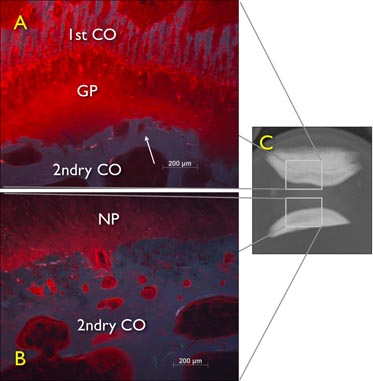
Figure 6. Free-swelling diffusion experiment of freshly prepared excised bovine tail intervertebral discs (IVDs) left for 24 hours in 1% procion red solution, demonstrating blockage effect by the growth plate. A digital X-ray sagittal view of a IVD still containing the growth plate and a second layer of bone fluorescent microscopy image of sagittal cut through the coccygeal bovine intervertebral disc explant with cartilaginous and bony endplate after 24h free diffusion. GP: growth plate, 2ndry CO: secondary center of ossification.

Figure 7. Free-swelling experiment of freshly prepared excised bovine tail intervertebral discs (IVDs) left for 24 hours in 1% procion red solution, demonstrating cleaning effect of jet lavage treatment. Sagittal thick sections (~100 mm) of excised bovine IVDs with ~1.5-mmthick bony end plates that underwent jet lavage treatment (Left) control side without treatment (Right) jet-lavaged treatment. Inlet shows the spray pattern, which was used from the Zimmer wound debridement system 24h free diffusion experiments of bovine intervertebral disc explants in 1% procion red.
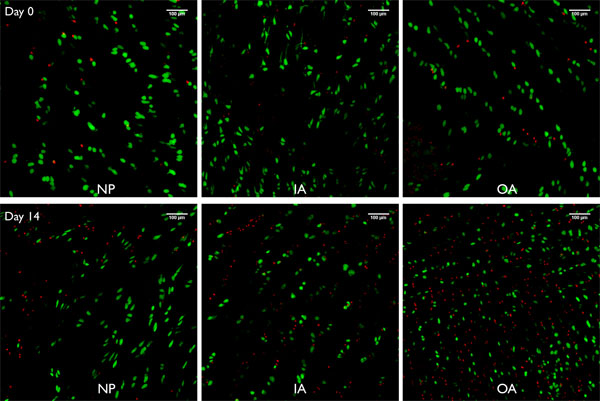
Figure 8. Live/Dead Images of projections of confocal Imaging stacks ( ̃250μm) taken from bovine intervertebral disc segments prepared with this jet-lavage method kept under free-swelling for 0 and 14 days respectively for the nucleus pulposus (NP), the inner annulus fibrosus (IA) and the outer annulus fibrosus (OA). Green cells = live cells stained by calcein AM (acetyl methylester), red cells = dead cells nucleus stained by ethidium homodimer-1.
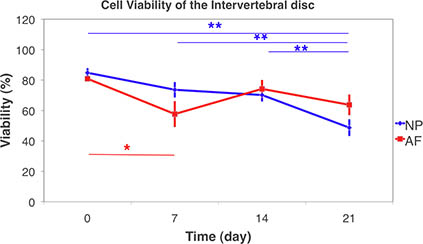
Figure 9. Cell Viability of intervertebral disc using confocal laser scanning microscopy on live tissue and 3D stack scanning. Cell viability of Nucleus Pulposus (NP) and Annulus Fibrosis (AF) over the 21-day-culture under free-swelling condition. (Mean ± SEM, N=6) Statistical differences were tested using non-parametric Kruskal-Wallis signed rank sum test among groups. Significant differences were found between day 21 and all other time points in NP. While in AF significant differences were found between day 7 to day 0. (* p < 0.05, ** p < 0.01).
Access restricted. Please log in or start a trial to view this content.
Dyskusje
The first step for successful organ culture is to make sure that the explant should not be contaminated. The tail should be skinned before you start with the procedure. Any animal hair brought into a sterile lab could be problematic in terms of contamination. The bovine tail should ideally be as fresh as possible (this affects initial cell viability). Furthermore, the betadine washing step is recommended to reduce the risk of contamination further. Instead of using a custom-made blade holder and a hammer to separate the ...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
We have nothing to disclose.
Podziękowania
This project was supported by the Swiss National Science Foundation (SNF # 310030-127586/1).
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| Fresh bovine intervertebral disc tissue from bovine tails, from local slaughter house (ideally within hours post-mortem and without skin). | |||
| Pulsavac Plus AC System | Zimmer inc., Switzerland | 00-5150-486-01 | Best performance with the hip-spray head and with AC power supply (the one with the 8 AA battery pack d–s also work but is less convenient) |
| High Capacity Fan Spray w/Splash Shield, 12.7cm length | Zimmer inc., Switzerland | 00-5150-175-00 | There are several spray heads available, we tested this one successfully |
| Scalpel blades #22 and #10 | Swann-Morton | #10: 0201 #22: 0208 | |
| Scalpel blade holder # 3 and #4 | Hausmann, Germany | #3: 06.103.00 #4: 06.104.00 | |
| Lutz industrial blade | Lutz, Germany | 1022.0884 | |
| Phosphate buffered Saline (PBS) | Invitrogen | 10010-023 | |
| Dulbecco’s Modified Eagle Medium (DMEM) | GIBCO, by Life Technologies | 11960-044 | |
| Lactated Ringer’s solution (without glucose) | Bichsel, Switzerland | 133 0002 | |
| 6-well multi-well plate | Techno Plastic Products | 92006 | |
| Betadine solution | Mundipharma, Switzerland | 10055025 | |
| Surgical skin marker | Porex Surgical, Switzerland | 9560 | |
| Large cutting board | Any brand is possible |
Odniesienia
- Lee, C. R., Iatridis, J. C., Poveda, L., Alini, M. In vitro organ culture of the bovine intervertebral disc: effects of vertebral endplate and potential for mechanobiology studies. Spine (Phila Pa 1976). 31, 515-522 (1976).
- Chan, S. C. W., Gantenbein-Ritter, B., Leung, V. Y., Chan, D. Cryopreserved intervertebral disc with injected bone marrow-derived stromal cells: a feasibility study using organ culture. Spine. J. 10 (6), 486-496 (2010).
- Chan, S. C., Ferguson, S. J., Wuertz, K., Gantenbein-Ritter, B. Biological Response of the Intervertebral Disc to Repetitive Short Term Cyclic Torsion. Spine (Phila Pa 1976). , (2011).
- Gantenbein-Ritter, B., Sprecher, C. M., Chan, S., Illien-Jünger, S., Grad, S. Confocal imaging protocols for live/dead staining in three-dimensional carriers. Methods Mol. Biol. 740, 127-140 (2011).
- Gantenbein-Ritter, B., Potier, E., Zeiter, S., van der Werf, M. Accuracy of three techniques to determine cell viability in 3D tissues or scaffolds. Tissue Engineering Part C Methods. 14, 353-358 (2008).
- Rasband, W. S. ImageJ. , National Institutes of Health. Bethesda, Maryland, USA. (1997).
- Haschtmann, D., Stoyanov, J. V., Ettinger, L., Nolte, L. P., Ferguson, S. J. Establishment of a novel intervertebral disc/endplate culture model: analysis of an ex vivo in vitro whole-organ rabbit culture system. Spine. 31, 2918-2925 (2006).
- Gantenbein, B., Grünhagen, T., Lee, C. R., van Donkelaar, C. C. An in vitro organ culturing system for intervertebral disc explants with vertebral endplates: a feasibility study with ovine caudal discs. Spine. 31, 2665-2673 (2006).
- Korecki, C. L., Kuo, C. K., Tuan, R. S., Iatridis, J. C. Intervertebral disc cell response to dynamic compression is age and frequency dependent. J. Orthop. Res. 27, 800-806 (2009).
- Korecki, C. L., MacLean, J. J., Iatridis, J. C. Dynamic compression effects on intervertebral disc mechanics and biology. Spine (Phila Pa 1976). 33, 1403-1409 (1976).
- Jim, B., Steffen, T., Moir, J., Roughley, P., Haglund, L. Development of an intact intervertebral disc organ culture system in which degeneration can be induced as a prelude to studying repair potential. European Spine Journal. , 1-11 (2011).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone