Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Modeling Colitis-Associated Cancer with Azoxymethane (AOM) and Dextran Sulfate Sodium (DSS)
W tym Artykule
Podsumowanie
We demonstrate a protocol in which administration of the genotoxic agent azoxymethane (AOM) followed by three cycles of the pro-inflammatory agent dextran sulfate sodium (DSS) rapidly and consistently generates colon tumors in mice with morphologic and molecular similarities to those seen in human colitis-associated cancer.
Streszczenie
Individuals with inflammatory bowel disease (IBD), such as Crohn's disease (CD) or ulcerative colitis (UC) are at increased risk of developing colorectal cancer (CRC) over healthy individuals. This risk is proportional to the duration and extent of disease, with a cumulative incidence as high as 30% in individuals with longstanding UC with widespread colonic involvement.1 Colonic dysplasia in IBD and colitis associated cancer (CAC) are believed to develop as a result of repeated cycles of epithelial cell injury and repair while these cells are bathed in a chronic inflammatory cytokine milieu.2 While spontaneous and colitis-associated cancers share the quality of being adenocarcinomas, the sequence of underlying molecular events is believed to be different.3 This distinction argues the need for specific animal models of CAC.
Several mouse models currently exist for the study of CAC. Dextran sulfate sodium (DSS), an agent with direct toxic effects on the colonic epithelium, can be administered in drinking water to mice in multiple cycles to create a chronic inflammatory state. With sufficient duration, some of these mice will develop tumors.4 Tumor development is hastened in this model if administered in a pro-carcinogenic setting. These include mice with genetic mutations in tumorigenesis pathways (APC, p53, Msh2), as well as mice pre-treated with genotoxic agents (azoxymethane [AOM], 1,2-dimethylhydrazine [DMH]).5
The combination of DSS with AOM as a model for colitis associated cancer has gained popularity for its reproducibility, potency, low price, and ease of use. Though they have a shared mechanism, AOM has been found to be more potent and stable in solution than DMH. While tumor development in other models generally requires several months, mice injected with AOM and subsequently treated with DSS develop adequate tumors in as little as 7-10 weeks.6, 7 Finally, AOM and DSS can be administered to mice of any genetic background (knock out, transgenic, etc.) without cross-breeding to a specific tumorigenic strain. Here, we demonstrate a protocol for inflammation-driven colonic tumorigenesis in mice utilizing a single injection of AOM followed by three seven-day cycles of DSS over a 10 week period. This model induces tumors with histological and molecular changes closely resembling those occurring in human CAC and provides a highly valuable model for the study of oncogenesis and chemoprevention in this disease.8
Protokół
1. Colitis-associated Cancer Induction
- Set aside cages of sex and age-matched 6-8 week old mice to be used for experimental and control groups. Mice can be individually labeled with tail markings or ear clips.
- On day 0, record baseline weights and inject each mouse intraperitoneally (IP) with 10 mg/kg of AOM working solution (1 mg/ml in isotonic saline, diluted from 10 mg/ml stock solution in dH2O kept at -20 deg C). Based on experience, this dose can be adjusted between 7-14 mg/kg and/or repeated early in the experiment.
Caution: AOM is a volatile genotoxic agent and should be handled carefully according to the accompanying MSDS. Dilutions should be prepared in a chemical hood, maintained on ice, and discarded following institution specific protocols.
- Make a 2.5% (2.5 g/100 ml) DSS solution in distilled water and pass through a 0.22 μm cellulose acetate filter by vacuum. This dose can be adjusted between 1-3.5% depending on mouse strain and environment. Once prepared, DSS solution can be kept refrigerated for up to 1 week.
- On day 7, supply DSS solution to mice as their drinking water. Approximately 250 ml/cage will be needed every time new DSS is provided for a maximum of 5 mice/cage; however, these are only estimations and will vary depending on the type of water bottles used in your animal facility.
- To provide a continuous supply of DSS for seven days, DSS solution should be replaced in clean bottles three times (every 2-3 days) during this period. Some investigators measure the amount of DSS consumed prior to replacing with new solution as a measure of exposure.
- On day 14, switch cages back to standard drinking water for two weeks.
- Repeat steps (1.4) - (1.6) on days 28 and 49 to provide a second and third cycle of DSS. A DSS "cycle" consists of one week of DSS in the drinking water followed by 2 weeks of regular (autoclaved) water.
2. Clinical Assessment Colitis and Tumor Progression
- Mice should be weighed/observed 2-3 times per week. Percent weight loss relative to baseline is used as a surrogate measure of colitis severity. Regular assessments of rectal bleeding, diarrhea, or prolapse can be made depending on experimental goals and reported according to various scoring systems.9, 10
- A weight loss of up to 10% along with 2-3 days of diarrhea and rectal bleeding can be expected in the week following a full cycle of DSS (daily observation may be useful during this immediate post-DSS period).
It is possible that some mice may not recover; mice losing greater than 20% of their weight are less likely to survive and may require early euthanasia. A single IP injection of 0.5 - 1.0 ml saline in such mice can be a useful supportive measure towards correcting fluid volume lost due to diarrhea.
3. Murine Endoscopy (Optional)
- Endoscopy following the second or third cycle of DSS can be performed to confirm tumor growth in vivo prior to sacrifice. As the AOM/DSS model is quite reliable in producing tumors, this step is generally not necessary. Only a few mice should evaluated using this method, as it has the potential to disrupt mouse tumors.11
- Anesthetize the mouse with inhaled or injectable sedation.
- After mouse is anesthetized, secure the mouse to a flat board with tape at the tail and chest.
- Advance the murine endoscope into the mouse rectum gently using saline to inflate and irrigate to improve intraluminal view and remove fecal contents. (Note that endoscopy can also be performed using air insufflation as an alternative to saline and this technique may offer improved visualization of the tumor.12)
4. Mouse Sacrifice and Colon Harvesting
- On day 70, each AOM/DSS treated mouse should harbor multiple colon tumors and be ready for assessment. This date can be delayed weeks to months if larger tumors are desired. (Of note, mice with severe rectal prolapsed may need to be euthanized early to avoid excessive discomfort to animals as per institutional animal care committees.)
- Prior to necropsy, individually euthanize mice with isoflurane and cervical dislocation (or other institutionally-approved method). Final weight and other measurements can be made at this time.
- Lay the mouse with its ventral side exposed on a cutting board. Cover the abdomen with 70% ethanol to prevent hairs from contaminating the abdominal contents during extraction of the colon.
- Use forceps to grasp the midline of the abdomen and make a small incision to expose the peritoneum.
- Extend this incision on each side of the abdomen along the costal margin.
- Use scissors to cut through the pelvis so that the colon can be harvested down to the anorectal junction. This is important because DSS colitis injury is greatest at the distal rectum and correspondingly, this is the region of greatest tumor development.
- Additional tissues such as mesenteric lymph nodes may also be harvested at this time.
5. Preparing the Colon for Macroscopic Analysis
- Using an 18G gavage needle attached to a 5 ml syringe, flush the colon with ice-cold phosphate-buffered saline (PBS) such that fluid exits in the physiologic direction. If colon tissue will be used for RNA or protein-based analysis, it can be kept cold by employing pre-chilled work trays.
- Open the flushed colon longitudinally along its mesentery and paint onto the cold tray. Tumors are more easily visualized when the colon is placed on a dark surface. Alternatively, 1% Alcian blue dye can be applied to highlight tumors. Assess tumor number and size with a ruler or digital caliper.
- Small sections of tumor and adjacent normal tissue can be excised at this point for future analysis by RNA, protein, or immunohistochemical (IHC) methods, leaving some portion of the remaining colon for histological assessment.
Digital photography of the gross colon specimens immediately following step 5.2 can be helpful for precise analysis of tumor burden, especially if significant portions of the colons are excised for other forms of analysis as described above. Tumor burden (%) can be calculated as tumor area/total colon area using free software such as ImageJ.
6. Preparing the Colon for Histological Assessment
- Apply 10% formalin to the remaining colon on the cold tray via syringe or finger. Allowing the tissue to fix for at least 30 sec in this manner facilitates transfer to the fixation basin. Ensure residual formalin is wiped off prior to painting a new opened colon on the same tray.
- Transfer colons to a basin filled with 10% formalin and pin down at edges.
- After 3 hr of fixation, transfer colons to 70% ethanol. Colons can be preserved in 70% ethanol indefinitely.
- Three longitudinal sections of colon (proximal, mid, distal/rectum) can be stabilized in 2% agar and submitted for paraffin embedding. Assure that the proximal colon and distal colon/rectum are oriented in a uniform manner for each mouse.
- Dysplasia, crypt damage, and inflammation can be described and assessed by an experienced individual according to previously published protocols.10, 13-15 Mice can be sacrificed prior to day 70 if the experimenter is interested in detecting early dysplastic events.
7. Representative Results
The AOM/DSS model described herein allows the researcher to reliably generate colon tumors in mice. Tumor growth in this model is directly influenced by the associated inflammatory process. Colitis severity should be monitored clinically by following weight loss and presence of diarrhea/hematochezia (Figure 2). These signs of disease activity tend to begin by day 5 of the DSS cycle and for four or more days after DSS is removed. Rarely, mice with a significant rectal tumor burden can develop rectal prolapse. After the second or third DSS cycle diarrhea may become persistent. Typically tumors are present and identifiable by murine colonoscopy prior to the third cycle of DSS (Figure 3). Additional time and a third course of DSS results in larger tumors at the time of harvest (Figure 4). Use of topically applied Alcian blue stain may be used to highlight tumors (Figure 5). Photographs of colonic tumors will assist in generating tumor measurements which can be used to quantitatively compare tumor production and size between experimental groups (Figure 6). Fixed and paraffin embedded colon specimens can then be evaluated for histology or with the use of immunohistochemical staining (Figure 7 and 8).
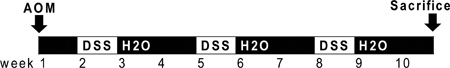
Figure 1. Schematic of AOM and DSS administration. AOM (10 mg/kg) is injected on day 0. At the beginning of the second week (day 7), 2.5% DSS solution is administered to mice in their drinking water. Seven days of DSS is followed by two weeks of autoclaved water. An additional two cycles of DSS are administered prior to sacrifice.

Figure 2. Mouse weight relative to baseline during AOM and DSS administration. Note that in the week following each DSS cycle, mice lose 5-10% of their body weight. Weight loss in this experiment is a surrogate marker for colitis severity.
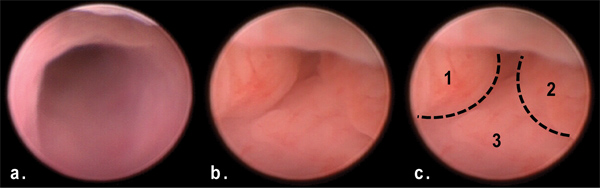
Figure 3. View of tumors in distal colon via murine endoscopy at day 50 of AOM/DSS treatment. Note the multiple polypoid masses obstructing the lumen of the distal colon (b, c) in comparison to the normal colon (a).
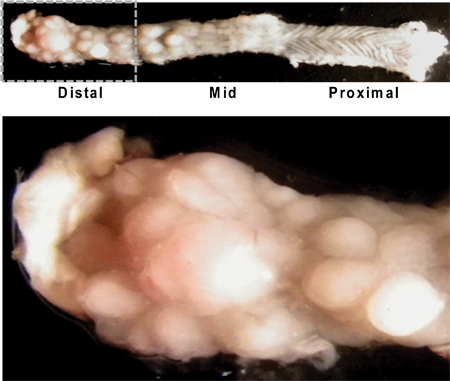
Figure 4. Longitudinally opened mouse colon illustrating gross appearance of tumors. Note the higher tumor burden in the distal colon/rectum (left upper image), and the characteristic rugated texture of the proximal colon (right upper image) with little tumor growth. A close up view of the distal colon shows numerous tumors of varying sizes (below).
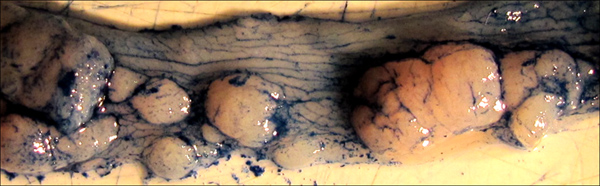
Figure 5. Tumors highlighted by application of Alcian blue stain. Note how the dye emphasizes the normal texture of the colon as well as the borders of each individual tumor. Such staining can be helpful in the precise measurement of tumor areas by ruler or digital measurement.
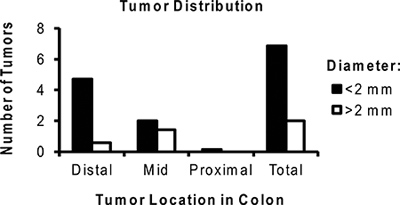
Figure 6. Representative distribution of the average number of tumors per mouse treated with AOM/DSS. Note the majority of tumors are located in the distal colon and are <2 mm in size.
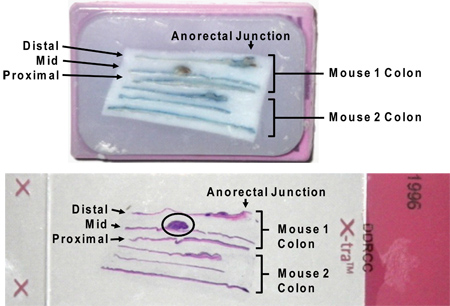
Figure 7. Paraffin-embedded longitudinal sections of colon in cassette (above) and on slide following H&E stain (below). Note that residual Alcian blue stain does not interfere with H&E staining. A large tumor is circled on the slide image (circled). The designations "distal," "middle," and "proximal" result from sectioning the entire colon in thirds between the cecum and anus.

Figure 8. Representative histology of a tumor resulting from AOM/DSS administration in the distal colon. H&E, BrdU, and β-catenin stained slides at 50X (Top panel) and 400X (Bottom panel) respectively demonstrate dysplastic changes similar to human adenocarcinomas of the colon.
Dyskusje
Treatment of mice with AOM and DSS rapidly and effectively models human colitis-associated cancer. Hypotheses regarding heritable factors contributing to colitis-associated cancers can be easily studied with genetically engineered mice.13, 16 Alternatively, the effect of pharmacologic targets in colitis-associated cancer can be studied by employing wild-type mice.
While this model is highly valued by those interested in the study of colon tumor development in the setting of inflamma...
Ujawnienia
No conflicts of interest declared.
Podziękowania
This work was funded in part by DK089016 and L30 RR030244 (MAC), CA153036 (AS), and P30-DK52574 (to the Washington University Digestive Diseases Research Core). A.I.T. was a Howard Hughes Medical Institute Medical Research Training Fellow.
Materiały
| Name | Company | Catalog Number | Comments |
| C57BL/6J Mice | Jackson Laboratory | 000664 | |
| Azoxymethane (AOM) | Sigma Aldrich | A5486-100MG | Stock solution: dilute to 10 mg/ml in distilled water to be kept at -20 °C as 0.5 - 1 ml aliquots. Working solution: dilute stock to 1 mg/ml in isotonic (0.9%) saline |
| Dextran Sulfate Sodium (DSS) | TdB Consultancy | DB001 | MW 40 kDa (36-50 kDa preparations from other sources are acceptable; The same lot should be used for a single experiment)6 |
| Coloview miniendoscopic system | Karl Storz | Multiple | See Becker et al. for detailed explanation of equipment and setup.11 |
| TPP Rapid FILTERMAX 500 ml Bottle-Filter, 0.22 μm PES | Midwest Scientific | TP99500 | Any standard tissue culture filter is acceptable |
| Ethyl Alcohol 200 Proof ASC/USP | Pharmaco-AAPER (or other) | 11ACS200 | Dilute to 70% in distilled water |
| Isoflurane, USP | Butler Animal Health Supply | 4029405 | Place mouse in glass jar with gauze or a small cloth soaked in anesthetic |
| 18G Straight Gavage Needle | Braintree Scientific | N-008 | |
| Phosphate Buffered Saline (PBS) | Sigma Aldrich | P5493 | Dilute to 1X (0.01 M) in distilled water |
| Cold Tray (Tissue Tek II Cold Plate) | Fisher Scientific | NC9491941 | Store at -20 °C |
| ImageJ Software | NIH (free download) | http://rsbweb.nih.gov/ij/ | |
| Formaldehyde (37%) | Fisher Scientific | F79-500 | Dilute to 10% in PBS |
| BD Bacto Agar | Fisher Scientific | DF0140-01-0 | Use hotplate to create 2% solution in distilled water |
| Miltex Eye Dressing Forceps | MedPlus Inc. | 18-780 | |
| Miltex Eye Scissors | MedPlus Inc. | 18-1430 | Curved points prevent damage to colon during opening. |
| Alcian Blue 8GX (powder) | Sigma Aldrich | A5268 | Add 1 g powder to 100 ml 3% acetic acid (3 ml glacial acetic acid + 97 ml distilled water) |
| 1 mL Tuberculin syringe with attached 26 G x 3/8 in intradermal bevel needle | BD | 305946 | For injection of AOM |
Odniesienia
- Ekbom, A. Ulcerative colitis and colorectal cancer. A population-based study. N. Engl. J. Med. 323, 1228-1233 (1990).
- Terzic, J. Inflammation and colon cancer. Gastroenterology. 138, 2101-2114 (2010).
- Ullman, T. A., Itzkowitz, S. H. Intestinal inflammation and cancer. Gastroenterology. 140, 1807-1816 (2011).
- Okayasu, I. Dysplasia and carcinoma development in a repeated dextran sulfate sodium-induced colitis model. J. Gastroenterol. Hepatol. 17, 1078-1083 (2002).
- Kanneganti, M., Mino-Kenudson, M., Mizoguchi, E. Animal models of colitis-associated carcinogenesis. J. Biomed. Biotechnol. 342637, (2011).
- Neufert, C., Becker, C., Neurath, M. F. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat. Protoc. 2, 1998-2004 (2007).
- Tanaka, T. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 94, 965-973 (2003).
- De Robertis, M. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J. Carcinog. 10, 9 (2011).
- Wirtz, S. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2, 541-546 (2007).
- Cooper, H. S. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 69, 238-249 (1993).
- Becker, C., Fantini, M. C., Neurath, M. F. High resolution colonoscopy in live mice. Nat. Protoc. 1, 2900-2904 (2006).
- Becker, C., Fantini, M. C., Wirtz, S., Nikolaev, A., Kiesslich, R., Lehr, H. A., Galle, P. R., Neurath, M. F. In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy. Gut. 54, 950-954 (2005).
- Shaker, A. Epimorphin deletion protects mice from inflammation-induced colon carcinogenesis and alters stem cell niche myofibroblast secretion. J. Clin. Invest. 120, 2081-2093 (2010).
- Boivin, G. P. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 124, 762-777 (2003).
- Cooper, H. S. Dysplasia and cancer in the dextran sulfate sodium mouse colitis model. Relevance to colitis-associated neoplasia in the human: a study of histopathology, B-catenin and p53 expression and the role of inflammation. Carcinogenesis. 21, 757-768 (2000).
- Yoshida, Y. The forkhead box M1 transcription factor contributes to the development and growth of mouse colorectal cancer. Gastroenterology. 132, 1420-1431 (2007).
- Suzuki, R. Strain differences in the susceptibility to azoxymethane and dextran sodium sulfate-induced colon carcinogenesis in mice. Carcinogenesis. 27, 162-169 (2006).
- Mahler, M. Differential susceptibility of inbred mouse strains to dextran sulfate sodium-induced colitis. Am. J. Physiol. 274, 544-551 (1998).
- Nambiar, P. R. Preliminary analysis of azoxymethane induced colon tumors in inbred mice commonly used as transgenic/knockout progenitors. Int. J. Oncol. 22, 145-150 (2003).
- Tanaka, T. Colorectal carcinogenesis: Review of human and experimental animal studies. J Carcinog. 8, (2009).
- Ciorba, M. A. Induction of IDO-1 by immunostimulatory DNA limits severity of experimental colitis. J. Immunol. 184, 3907-3916 (2010).
- Kerr, T. A. Dextran sodium sulfate inhibition of real-time polymerase chain reaction amplification: A poly-A purification solution. Inflamm. Bowel Dis. 18, 344-348 (2012).
- Tang, Y. is required for resection-induced changes in apoptosis, proliferation, and members of the extrinsic cell death pathways. Gastroenterology. 126, 220-230 (2004).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone