Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Conducting Miller-Urey Experiments
W tym Artykule
Podsumowanie
The Miller-Urey experiment was a pioneering study regarding the abiotic synthesis of organic compounds with possible relevance to the origins of life. Simple gases were introduced into a glass apparatus and subjected to an electric discharge, simulating the effects of lightning in the primordial Earth’s atmosphere-ocean system. The experiment was conducted for one week, after which, the samples collected from it were analyzed for the chemical building blocks of life.
Streszczenie
In 1953, Stanley Miller reported the production of biomolecules from simple gaseous starting materials, using an apparatus constructed to simulate the primordial Earth's atmosphere-ocean system. Miller introduced 200 ml of water, 100 mmHg of H2, 200 mmHg of CH4, and 200 mmHg of NH3 into the apparatus, then subjected this mixture, under reflux, to an electric discharge for a week, while the water was simultaneously heated. The purpose of this manuscript is to provide the reader with a general experimental protocol that can be used to conduct a Miller-Urey type spark discharge experiment, using a simplified 3 L reaction flask. Since the experiment involves exposing inflammable gases to a high voltage electric discharge, it is worth highlighting important steps that reduce the risk of explosion. The general procedures described in this work can be extrapolated to design and conduct a wide variety of electric discharge experiments simulating primitive planetary environments.
Wprowadzenie
The nature of the origins of life on Earth remains one of the most inscrutable scientific questions. In the 1920s Russian biologist Alexander Oparin and British evolutionary biologist and geneticist John Haldane proposed the concept of a "primordial soup"1,2, describing the primitive terrestrial oceans containing organic compounds that may have facilitated chemical evolution. However, it wasn't until the 1950s when chemists began to conduct deliberate laboratory studies aimed at understanding how organic molecules could have been synthesized from simple starting materials on the early Earth. One of the first reports to this end was the synthesis of formic acid from the irradiation of aqueous CO2 solutions in 19513.
In 1952, Stanley Miller, then a graduate student at the University of Chicago, approached Harold Urey about doing an experiment to evaluate the possibility that organic compounds important for the origin of life may have been formed abiologically on the early Earth. The experiment was conducted using a custom-built glass apparatus (Figure 1A) designed to simulate the primitive Earth. Miller's experiment mimicked lightning by the action of an electric discharge on a mixture of gases representing the early atmosphere, in the presence of a liquid water reservoir, representing the early oceans. The apparatus also simulated evaporation and precipitation through the use of a heating mantle and a condenser, respectively. Specific details about the apparatus Miller used can be found elsewhere4. After a week of sparking, the contents in the flask were visibly transformed. The water turned a turbid, reddish color5 and yellow-brown material accumulated on the electrodes4. This groundbreaking work is considered to be the first deliberate, efficient synthesis of biomolecules under simulated primitive Earth conditions.
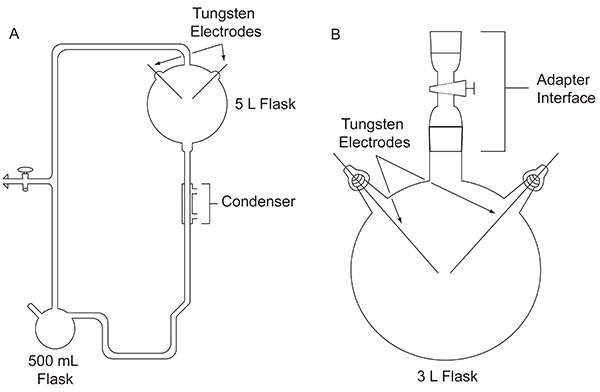
Figure 1. Comparison between the two types of apparatuses discussed in this paper. The classic apparatus used for the original Miller-Urey experiment (A) and the simplified apparatus used in the protocol outlined here (B). Click here to view larger image.
After the 1953 publication of results from Miller's classic experiment, numerous variations of the spark discharge experiment, for example using other gas mixtures, were performed to explore the plausibility of producing organic compounds important for life under a variety of possible early Earth conditions. For example, a CH4/H2O/NH3/H2S gas mixture was tested for its ability to produce the coded sulfur-containing α-amino acids, although these were not detected6. Gas chromatography-mass spectrometry (GC-MS) analysis of a CH4/NH3 mixture subjected to an electric discharge showed the synthesis of α-aminonitriles, which are amino acid precursors7. In 1972, using a simpler apparatus, first introduced by Oró8 (Figure 1B), Miller and colleagues demonstrated the synthesis of all of the coded α-amino acids9 and nonprotein amino acids10 that had been identified in the Murchison meteorite to date, by subjecting CH4, N2, and small amounts of NH3 to an electric discharge. Later, using this same simplified experimental design, gas mixtures containing H2O, N2, and CH4, CO2, or CO were sparked to study the yield of hydrogen cyanide, formaldehyde, and amino acids as a function of the oxidation state of atmospheric carbon species11.
In addition to the exploration of alternative experimental designs over the years, significant analytical advances have occurred since Miller's classic experiment, which recently aided more probing investigations of electric discharge experimental samples archived by Miller, than would have been facilitated by the techniques Miller had access to in the 1950s. Miller's volcanic experiment12, first reported in 19554, and a 1958 H2S-containing experiment13 were shown to have formed a wider variety, and greater abundances, of numerous amino acids and amines than the classic experiment, including many of which that had not been previously identified in spark discharge experiments.
The experiment described in this paper can be conducted using a variety of gas mixtures. Typically, at the very least, such experiments will contain a C-bearing gas, an N-bearing gas, and water. With some planning, almost any mixture of gases can be explored, however, it is important to consider some chemical aspects of the system. For example, the pH of the aqueous phase can have a significant impact on the chemistry that occurs there14.
The method described here has been tailored to instruct researchers how to conduct spark discharge experiments that resemble the Miller-Urey experiment using a simplified 3 L reaction vessel, as described in Miller's 1972 publications9,10. Since this experiment involves a high voltage electric arc acting on inflammable gases, it is crucial to remove O2 from the reaction flask to eliminate the risk of explosion, which can occur upon combustion of reduced carbon-bearing gases such as methane or carbon monoxide, or reaction of H2 with oxygen.
There are additional details that should be kept in mind when preparing to conduct the experiment discussed here. First, whenever working with glass vacuum lines and pressurized gases, there exists the inherent danger of both implosion and over-pressuring. Therefore, safety glasses must be worn at all times. Second, the experiment is typically conducted at less than atmospheric pressure. This minimizes the risk of over-pressuring the manifold and reaction flask. Glassware may be rated at or above atmospheric pressure, however, pressures above 1 atm are not recommended. Pressures may increase in these experiments as water-insoluble H2 is liberated from reduced gases (such as CH4 and NH3). Over-pressuring can lead to seal leakage, which can allow atmospheric O2 to enter the reaction flask, making it possible to induce combustion, resulting in an explosion. Third, it should be borne in mind that modification of this protocol to conduct variations of the experiment requires careful planning to ensure unsafe conditions are not created. Fourth, it is highly recommended that the prospective experimenter read through the entire protocol carefully several times prior to attempting this experiment to be sure he or she is familiar with potential pitfalls and that all necessary hardware is available and in place. Lastly, conducting experiments involving combustible gases require compliance with the experimenter's host institution's Environmental Health and Safety departmental guidelines. Please observe these recommendations before proceeding with any experiments. All steps detailed in the protocol here are in compliance with the authors' host institutional Environmental Health and Safety guidelines.
Protokół
1. Setting Up a Manifold/Vacuum System
- Use a glass manifold to introduce gases into the reaction flask. This manifold can be purchased or constructed by a glass-blowing facility, but must include vacuum-tight ports that can be connected to a vacuum system, gas cylinders, a vacuum gauge, and the reaction vessel.
- Use ground glass joints and glass plugs with valves on the manifold. Ensure that all O-rings on the plugs are capable of making the necessary seals. If using glass joints, a sufficient amount of vacuum grease can be applied to help make a seal, if necessary. Silicon vacuum grease can be used to avoid potential organic contamination.
- Use glass stopcocks on the manifold. Apply the minimum amount of vacuum grease necessary to make a seal.
- Measure the manifold volume. This volume will be used for calculations related to final gas pressures in the 3 L reaction flask and should be known as precisely as possible.
- Unless the manifold has enough connections to accommodate all gas cylinders simultaneously, connect one cylinder at a time to the manifold. Include in this connection, a tap allowing the manifold to be isolated from the ambient atmosphere.
- Use suitable, clean, inert, and chemical and leak resistant tubing and ultratorr vacuum fittings to connect the gas cylinders to the manifold. Ultratorr fittings, where used, are to be finger-tightened.
- Connect to the manifold, a vacuum pump capable of establishing a vacuum of <1 mmHg. The vacuum pump exhaust should be located within the fume hood, or properly vented by other means.
- To ensure rapid attainment of vacuum and to protect the pump, insert a trap between the manifold and the vacuum pump. A liquid nitrogen finger-trap is recommended as it will prevent volatiles such as NH3, CO2, and H2O from entering the pump. Care should be taken, as trapped volatiles, upon warming, may overpressure the manifold and result in glass rupture.
- Connect to the manifold, a manometer or other vacuum gauge capable of 1 mmHg resolution or better. While various devices can be used, a mercury manometer, or MacLeod gauge, is preferable as mercury is fairly nonreactive.
- Measure and record the ambient temperature using a suitable thermometer.
2. Preparation of Reaction Flask
- Heat all glassware at 500 °C for at least 3 hr in air prior to use, to remove organic contaminants.
- Clean the tungsten electrodes by gently washing with clean laboratory wipes and methanol, and drying in air.
- Pour 200 ml of ultrapure water (18.2 MΩ cm, <5 ppb TOC) into the 3 L reaction flask.
- Introduce a precleaned and sterilized magnetic stir bar, which will ensure rapid dissolution of soluble gases and mixing of reactants during the experiment.
- Attach the tungsten electrodes to the 3 L reaction flask using a minimal amount of vacuum grease, with tips separated by approximately 1 cm inside the flask. Fasten with clips.
- Insert an adapter with a built-in stopcock into the neck of the 3 L reaction flask and secure with a clip.
- Attach the 3 L reaction flask to the gas manifold via the adapter. Use a clip or clamp to help secure the flask.
- Lightly grease all connections to ensure a good vacuum seal.
- Open all valves and stopcocks on the manifold, except Valve 6 and Stopcock 1 (Figure 4), and turn on the vacuum pump to evacuate the manifold. Once a stable vacuum reading of <1 mmHg has been attained, close Valve 1 and allow the manifold to sit for ~15 min to check for vacuum leaks. If none are detected, proceed to step 2.8. Otherwise troubleshoot the various connections until the leaks can be identified and fixed.
- Apply magnetic stirring to the reaction vessel. Open Valve 1 and Stopcock 1 (Figure 4) to evacuate the headspace of the 3 L reaction flask until the pressure has reached <1 mmHg.
- Close Valve 1 (Figure 4) and monitor the pressure inside the 3 L reaction flask. The measured pressure should increase to the vapor pressure of water. To ensure that no leaks exist, wait ~5 min at this stage. If the pressure (as read on the manometer) increases while Valve 1 is closed during this step, check for leaks in Stopcock 1 and the various reaction flask connections. If no leak is found, proceed to the next step.
3. Introduction of Gaseous NH3
- Calculate the necessary pressure of gaseous NH3 to introduce into the manifold such that 200 mmHg of NH3 will be introduced into the reaction flask. Details on how to do this are provided in the Discussion section.
- Close Valves 1 and 6, and Stopcock 1 (Figure 4) before introducing any gas into the manifold. Leave the other valves and stopcock open.
- Introduce NH3 into the manifold until a small pressure (approximately 10 mmHg) is reached and then evacuate the manifold to a pressure of <1 mmHg by opening Valve 1 (Figure 4). Repeat 3x.
- Introduce NH3 into the manifold to reach the pressure determined in step 3.1.
- Open Stopcock 1 (Figure 4) to introduce 200 mmHg of NH3 into the 3 L reaction flask. The NH3 will dissolve in the water in the reaction flask and the pressure will fall slowly.
- Once the pressure stops dropping, close Stopcock 1 (Figure 4) and record the pressure read by the manometer. This value represents the pressure inside the flask and will be used to calculate the pressures for other gases that will be introduced into the manifold later.
- Open Valve 1 (Figure 4) to evacuate the manifold to a pressure of <1 mmHg.
- Close Valve 2 (Figure 4) and disconnect the NH3 gas cylinder from the manifold.
4. Introduction of CH4
- Calculate the necessary pressure of CH4 to be introduced into the manifold such that 200 mmHg of CH4 will be introduced into the 3 L reaction flask. Example calculations are shown in the Discussion section.
- Connect the CH4 gas cylinder to the manifold.
- Open all valves and stopcocks, except Valve 6 and Stopcock 1 (Figure 4), and evacuate the manifold to a pressure of <1 mmHg.
- Close Valve 1 once the manifold has been evacuated (Figure 4).
- Introduce CH4 into the manifold until a small pressure (approximately 10 mmHg) is obtained. This purges the line of any contaminant gases from preceding steps. Open Valve 1 (Figure 4) to evacuate the manifold to <1 mmHg. Repeat 2x more.
- Introduce CH4 into the manifold until the pressure calculated in step 4.1, is reached.
- Open Stopcock 1 (Figure 4) to introduce 200 mmHg of CH4 into the 3 L reaction flask.
- Close Stopcock 1 once the intended pressure of CH4 has been introduced into the 3 L reaction flask (Figure 4) and record the pressure measured by the manometer.
- Open Valve 1 (Figure 4) to evacuate the manifold to <1 mmHg.
- Close Valve 2 (Figure 4) and disconnect the CH4 cylinder from the manifold.
5. Introduction of Further Gases (e.g. N2)
- At this point, it is not necessary to introduce additional gases. However, if desired, it is recommended to add 100 mmHg of N2. In this case, calculate the necessary pressure of N2 to be introduced into the manifold such that 100 mmHg of N2 will be introduced into the 3 L reaction flask. Example calculations are shown in the Discussion section.
- Connect the N2 gas cylinder to the manifold.
- Open all valves and stopcocks, except Valve 6 and Stopcock 1 (Figure 4), and evacuate the manifold to a pressure of <1 mmHg.
- Close Valve 1 once the manifold has been evacuated (Figure 4).
- Introduce N2 into the manifold until a small pressure (approximately 10 mmHg) is obtained. Open Valve 1 (Figure 4) to evacuate the manifold to <1 mmHg. Repeat 2x more.
- Introduce N2 into the manifold until the pressure calculated in step 5.1 is reached.
- Open Stopcock 1 (Figure 4) to introduce 100 mmHg of N2 into the reaction flask.
- Close Stopcock 1 once the intended pressure of N2 has been introduced into the reaction flask, (Figure 4) and record the pressure using the manometer.
- Open Valve 1 (Figure 4) to evacuate the manifold to <1 mmHg.
- Close Valve 2 (Figure 4) and disconnect the N2 cylinder from the manifold.
6. Beginning the Experiment
- Detach the reaction flask from the manifold by closing Stopcock 1 and Valve 1 (Figure 4) once all gases have been introduced into the reaction flask, so that ambient air may enter the manifold and bring the manifold up to ambient pressure.
- After carefully disconnecting the reaction flask from the manifold, set the flask somewhere it will not be disturbed (e.g. inside an empty fume hood).
- Disconnect the vacuum pump and carefully remove the cold trap and allow venting inside a fully operational fume hood.
- Secure the Tesla coil connected to the high frequency spark generator.
- Connect the opposite tungsten electrode to an electrical ground to enable the efficient passage of electrical current across the gap between the two electrodes.
- Set the output voltage of the spark generator to approximately 30,000 V, as detailed by documents available from the manufacturer.
- Prior to initiating the spark, close the fume hood sash, to serve as a safety shield between the apparatus and the experimenter. Turn the Tesla coil on to start the experiment, and allow sparking to continue for 2 weeks (or other desired period) in 1 hr on/off cycles.
7. End of Experiment
- Stop the experiment by turning off the Tesla coil.
- Open Stopcock 1 (Figure 4) to slowly introduce ambient air into the reaction flask and facilitate the removal of the adapter and the tungsten electrodes so samples can be collected. If desired, a vacuum can be used to evacuate the reaction flask of noxious reaction gases.
8. Collecting Liquid Sample
- Using a pyrolyzed glass pipette, remove liquid samples from the reaction flask, being careful to minimize exposure to contaminants, such as those that might be introduced by touching the pipette to the vacuum grease or other nonsterile surfaces.
- Transfer the sample to a sterile plastic or glass receptacle. Plastic receptacles are less prone to cracking or breaking upon freezing, compared to glass receptacles.
- Seal sample containers and store in a freezer capable of reaching temperatures of -20 °C or lower, as insoluble products may prevent the sample solution from freezing at 0 °C.
9. Cleaning the Apparatus
- Use clean laboratory wipes to carefully remove vacuum grease from the neck of the apparatus, the adapter and stopcock, and the glass surrounding the tungsten electrodes.
- Thoroughly clean the same surfaces described in step 9.1 with toluene to fully remove organic vacuum grease from the glassware. If using silicon grease, the high vacuum grease may remain on the glassware after pyrolysis, creating future problems, as detailed in the Discussion section.
- Thoroughly clean the reaction flask with a brush and the following solvents in order: ultrapure water (18.2 MΩ cm, <5 ppb TOC), ultrapure water (18.2 MΩ cm, <5 ppb TOC) with 5% cleaning detergent, methanol, toluene, methanol, ultrapure water (18.2 MΩ cm, <5 ppb TOC) with 5% cleaning detergent, and finally ultrapure water (18.2 MΩ cm, <5 ppb TOC).
- Cover all open orifices of the reaction flask with aluminum foil and wrap the adapter and its components in aluminum foil.
- Once all the glassware has been wrapped in aluminum foil, pyrolyze for at least 3 hr in air at 500 °C.
- Gently clean electrodes with methanol and let air dry.
10. Sample Analysis
Note: When preparing samples for analysis, the use of an acid hydrolysis protocol such as has been described in detail elsewhere15, is useful for obtaining more amino acids. Hydrolysis of a portion of the recovered sample provides the opportunity to analyze both free amino acids as well as their acid-labile precursors that are synthesized under abiotic conditions.
- For amino acid analysis, use a suitable technique (such as liquid chromatography and mass spectrometry-based methods, or other appropriate approaches). Such analytical techniques include high performance liquid chromatography with fluorescence detection (HPLC-FD)14, and ultrahigh performance liquid chromatography with fluorescence detection in parallel with time-of-flight positive electrospray ionization mass spectrometry (UHPLC-FD/ToF-MS)12,13. This manuscript describes analysis using mass spectrometric analyses via a triple quadrupole mass spectrometer (QqQ-MS) in conjunction with HPLC-FD.
Wyniki
The products synthesized in electric discharge experiments can be quite complex, and there are numerous analytical approaches that can be used to study them. Some of the more commonly used techniques in the literature for analyzing amino acids are discussed here. Chromatographic and mass spectrometric methods are highly informative techniques for analyzing the complex chemical mixtures produced by Miller-Urey type spark discharge experiments. Amino acid analyses can be conducted using o-phthaldialdehyde/N-acetyl...
Dyskusje
Numerous steps in the protocol described here are critical for conducting Miller-Urey type experiments safely and correctly. First, all glassware and sample handling tools that will come in contact with the reaction flask or sample need to be sterilized. Sterilization is achieved by thoroughly rinsing the items in question with ultrapure water (18.2 MΩ cm, <5 ppb TOC) and then wrapping them in aluminum foil, prior to pyrolyzing at 500 °C in air for at least 3 hr. Once the equipment has been pyrolyzed and while prepa...
Ujawnienia
The authors declare no competing financial interests.
Podziękowania
This work was jointly supported by the NSF and NASA Astrobiology Program, under the NSF Center for Chemical Evolution, CHE-1004570, and the Goddard Center for Astrobiology. E.T.P. would like to acknowledge additional funding provided by the NASA Planetary Biology Internship Program. The authors also want to thank Dr. Asiri Galhena for invaluable help in setting up the initial laboratory facilities.
Materiały
| Name | Company | Catalog Number | Comments |
| Glass Plugs for Manifold | Chemglass | CG-983-01 | |
| High Vacuum Grease | Apiezon | N/A | Type M/N |
| Silicon High Vacuum Grease | Dow Corning | 1597418 | |
| Teflon PFA Tubing | McMaster-Carr | 51805K54 | |
| Ultra-Torr Vacuum Fittings | Swagelok | SS-4-UT-6 | |
| Dry Scroll Vacuum Pump | Edwards | A72401905 | |
| U-Tube Manometer | Alta-Robbins | 100SS | |
| Tungsten Electrodes | Diamond Ground Products | TH2-1/16 | 2% thoriated |
| Methanol | Alfa Aesar | N/A | Ultrapure HPLC Grade |
| Teflon-Coated Magnetic Stir Bar | McMaster-Carr | 5678K127 | |
| Gaseous NH3 | Airgas | AMAHLB | 99.99% purity |
| Gaseous CH4 | Airgas | ME UHP300 | 99.99% purity |
| Gaseous N2 | Airgas | NI UHP300 | 99.999% purity |
| Tesla Coil | Electro-Technic Products | 15001 | Model BD-50E |
| 24 hr Plug-in Basic Timer | General Electric Company | 15119 | |
| Cleaning Detergent | Alconox | 1104 | |
| Toluene | Thermo Fisher Scientific | N/A | Optima Grade |
| Luna Phenyl-Hexyl HPLC Column | Phenomenex | 00G-4257-E0 | Brand: Luna |
| Formic Acid | Sigma-Alrich | F0507 | Used to make 50 mM ammonium formate |
Odniesienia
- Oparin, A. I. . The Origin of Life. , (1924).
- Haldane, J. B. The origin of life. Rationalist Annu. 148, 3-10 (1929).
- Garrison, W. M., Morrison, D. C., Hamilton, J. G., Benson, A. A., Calvin, M. Reduction of Carbon Dioxide in Aqueous Solutions by Ionizing Radiation. Science. 114, 416-418 (1951).
- Miller, S. L. Production of Some Organic Compounds under Possible Primitive Earth Conditions. J. Am. Chem. Soc. 77, 2351-2361 (1955).
- Miller, S. L. A Production of Amino Acids Under Possible Primitive Earth Conditions. Science. 117, 528-529 (1953).
- Heyns, H. K., Walter, W., Meyer, E. Model experiments on the formation of organic compounds in the atmosphere of simple gases by electrical discharges (Translated from German). Die Naturwissenschaften. 44, 385-389 (1957).
- Ponnamperuma, C., Woeller, F. α-Aminonitriles formed by an electric discharge through a mixture of anhydrous methane and ammonia. Biosystems. 1, 156-158 (1967).
- Oró, J. Synthesis of Organic Compounds by Electric Discharges. Nature. 197, 862-867 (1963).
- Ring, D., Wolman, Y., Friedmann, N., Miller, S. L. Prebiotic Synthesis of Hydrophobic and Protein Amino Acids. Proc. Natl. Acad. Sci. U.S.A. 69, 765-768 (1972).
- Wolman, Y., Haverland, W. J., Miller, S. L. Nonprotein Amino Acids from Spark Discharges and Their Comparison with the Murchison Meteorite Amino Acids. Proc. Natl. Acad. Sci. U.S.A. 69, 809-811 (1972).
- Roscoe, S., Miller, S. L. Energy Yields for Hydrogen Cyanide and Formaldehyde Syntheses: The HCN and Amino Acid Concentrations in the Primitive Ocean. Orig. Life. 17, 261-273 (1987).
- Johnson, A. P., et al. The Miller Volcanic Spark Discharge Experiment. Science. 322, 404 (2008).
- Parker, E. T., et al. Primordial synthesis of amines and amino acids in a 1958 Miller H2S-rich spark discharge experiment. Proc. Natl. Acad. Sci. U.S.A. 108, 5526-5531 (2011).
- Cleaves, H. J., Chalmers, J. H., Lazcano, A., Miller, S. L., Bada, J. L. A reassessment of prebiotic organic synthesis in neutral planetary atmospheres. Orig. Life Evol. Biosph. 38, 105-115 (2008).
- Glavin, D. P., et al. Amino acid analyses of Antarctic CM2 meteorites using liquid chromatography-time of flight-mass spectrometry. Meteorit. Planet. Sci. 41, 889-902 (2006).
- Zhao, M., Bada, J. L. Determination of α-dialkylamino acids and their enantiomers in geologic samples by high-performance liquid chromatography after a derivatization with a chiral adduct of o-phthaldialdehyde. J. Chromatogr. A. 690, 55-63 (1995).
- Strecker, A. About the artificial formation of lactic acid and a new Glycocoll the homologous body Justus Liebigs Annalen der Chemie. 75, 27-45 (1850).
- Miyakawa, S., Yamanashi, H., Kobayashi, K., Cleaves, H. J., Miller, S. L. Prebiotic synthesis from CO atmospheres: implications for the origins of life. Proc. Natl. Acad. Sci. U.S.A. 99, 14628-14631 (2002).
- Kobayashi, K., Kaneko, T., Saito, T., Oshima, T. Amino Acid Formation in Gas Mixtures by Particle Irradiation. Orig. Life Evol. Biosph. 28, 155-165 (1998).
- Sagan, C., Khare, B. N. Long-Wavelength Ultraviolet Photoproduction of Amino Acids on the Primitive Earth. Science. 173, 417-420 (1971).
- Harada, K., Fox, S. W. Thermal Synthesis of Natural Amino-Acids from a Postulated Primitive Terrestrial Atmosphere. Nature. 201, 335-336 (1964).
- Ponnamperuma, C., Lemmon, R. M., Mariner, R., Calvin, M. Formation of Adenine by Electron Irradiation of Methane Ammonia, and Water. Proc. Natl. Acad. Sci. USA. 49, 737-740 (1963).
- Bar-Nun, A., Bar-Nun, N., Bauer, S. H., Sagan, C. Shock Synthesis of Amino Acids in Simulated Primitive Environments. Science. 168, 470-473 (1970).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone