Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Generation of Human Alloantigen-specific T Cells from Peripheral Blood
W tym Artykule
Podsumowanie
This article describes a method for the generation and propagation of human T cell clones that specifically respond to a defined alloantigen. This protocol can be adapted for cloning human T cells specific for a variety of peptide-MHC ligands.
Streszczenie
The study of human T lymphocyte biology often involves examination of responses to activating ligands. T cells recognize and respond to processed peptide antigens presented by MHC (human ortholog HLA) molecules through the T cell receptor (TCR) in a highly sensitive and specific manner. While the primary function of T cells is to mediate protective immune responses to foreign antigens presented by self-MHC, T cells respond robustly to antigenic differences in allogeneic tissues. T cell responses to alloantigens can be described as either direct or indirect alloreactivity. In alloreactivity, the T cell responds through highly specific recognition of both the presented peptide and the MHC molecule. The robust oligoclonal response of T cells to allogeneic stimulation reflects the large number of potentially stimulatory alloantigens present in allogeneic tissues. While the breadth of alloreactive T cell responses is an important factor in initiating and mediating the pathology associated with biologically-relevant alloreactive responses such as graft versus host disease and allograft rejection, it can preclude analysis of T cell responses to allogeneic ligands. To this end, this protocol describes a method for generating alloreactive T cells from naive human peripheral blood leukocytes (PBL) that respond to known peptide-MHC (pMHC) alloantigens. The protocol applies pMHC multimer labeling, magnetic bead enrichment and flow cytometry to single cell in vitro culture methods for the generation of alloantigen-specific T cell clones. This enables studies of the biochemistry and function of T cells responding to allogeneic stimulation.
Wprowadzenie
T lymphocytes are critical components of the adaptive immune system. T cells are responsible for not only directly mediating protective immune responses to pathogens through a variety of effector mechanisms, but also actively maintaining immunological self-tolerance and directing the responses of other cells in the immune system. These functions are directed through a number of integrated signals, including T cell receptor (TCR) ligation, cytokines and chemokines, and metabolites1. Of these signals, the TCR is of particular importance, as it provides the characteristic specificity that defines the T cell’s role in adaptive immunity. A TCR interacts with linear peptide antigens presented by MHC (human ortholog HLA) molecules (pMHC complexes) in a highly specific and sensitive manner to provide the signals that initiate T cell effector function. The biochemical parameters of TCR interactions with pMHC ligands provide not only the specificity for T cell activation, but also have a qualitative impact on subsequent T cell function2. Thus, studying T cell function often requires examining the responses of clonal T cells with defined antigenic specificity.
The human T cell compartment, containing approximately 1012αβ T cells, contains an estimated 107– 108 distinct αβTCRs3-4. This diverse repertoire provides opportunity for recognition of the vast array of peptides from potential pathogens that would necessitate a T cell response for protective immunity. It is estimated that the frequency of T cells responding to a given foreign antigen presented by self-MHC is on the order of 10-4– 10-7 in the absence of prior immune response to that antigen5. The naive T cell repertoire is shaped by thymic selection to ensure the ability to recognize self-MHC presenting peptide antigens and limit reactivity against self-peptide antigens, maximizing the potential utility for mediating protective immunity2. However, in violation of this designed reactivity, a relatively large frequency, 10-3– 10-4, of T cells from immunologically naive individuals respond to stimulation with allogeneic cells, recognizing both the foreign MHC molecules as well as the endogenous peptides they present6. The recognition of allogeneic pMHC ligands is structurally similar to the recognition of foreign antigens presented by self-MHC; the TCR makes critical biochemical interactions with both the allogeneic MHC molecule as well as the presented peptide7. The robust nature of the response of T cells to allogeneic stimulation results from the diversity of pMHC complexes present on the surface of allogeneic cells8. It is estimated that each MHC presents approximately 2 x 104 different endogenous peptide antigens9. This breadth of response to allogeneic stimulation is a significant aspect of the clinically-relevant pathology, such as allograft rejection or graft versus host disease (GVHD), resulting from T cell alloreactivity.
Study of human T cell alloreactive responses has traditionally relied upon examining polyclonal responses of naive T cells following stimulation with allogeneic cells. Repeated stimulation with the same allogeneic cell line combined with limiting dilution analyses is capable of generating clonal T cells with defined recognition of allogeneic HLA10. However, this approach is problematic for examining responses to individual allogeneic pMHC ligands, as the large and diverse repertoire of endogenous pMHC complexes present for a given allogeneic HLA stimulates a broad repertoire of T cells. This bulk population stimulation and limiting dilution approach would require screening of large numbers of clones to isolate T cells with the desired reactivity against a single pMHC ligand. Additionally, the frequency of T cells responding to an individual allogeneic pMHC ligand is relatively low among naive T cell populations, which presents a barrier to efficient generation of human T cell clones responsive to a given antigen.
Identification and isolation of antigen-specific T cells from polyclonal populations have been enabled by the development of fluorophore-labeled pMHC multimers11. This approach utilizes specific peptide antigens loaded into recombinant soluble biotinylated MHC molecules, which are labeled by binding to a streptavidin-labeled fluorophore. Multimerization of pMHC increases the avidity, compensating for the intrinsically low (µM) affinity of TCR for soluble pMHC ligands. Labeled cells can be identified and isolated by flow cytometry. However, this approach is still limited by the low frequency of antigen-specific T cells among naive T cell populations, which are typically orders of magnitude less than the limit of accurate identification and quantification on most flow cytometers. To address this limitation, a method of pMHC tetramer labeling and subsequent magnetic bead enrichment for tetramer-labeled cells has been developed12. This method has demonstrated reliable detection, enumeration, and isolation of low-frequency antigen-specific T cells.
This protocol describes an effective protocol for the generation of human T cell clones that specifically respond to individual allogeneic pMHC ligands. The protocol applies pMHC (HLA) multimer labeling and enrichment for the isolation of alloantigen-specific human T cells with flow cytometry cell sorting and a robust method for in vitro culture of human T cells to enable production of T cell clones from single sorted cells (overview in Figure 1).
Protokół
NOTE: This protocol requires use of peripheral blood samples from human volunteers. All research with human subjects should be reviewed and approved by a Human Studies Institutional Review Board to ensure compliance with the Declaration of Helsinki (2013) and the Health Insurance Portability and Accountability Act of 1996.
1. Isolation of T cells from Whole Blood
- Prior to starting, warm the density gradient medium to room temperature. Aliquot 4 ml of density gradient medium into 2-4 sterile 15 ml conical centrifuge tubes (1 tube will be used for each 10 ml total volume of diluted blood).
- Obtain 10-20 ml of blood in 1-2 sodium heparin spray coated (green-top) venous blood collection tubes. Collect human specimens under the supervision of trained individuals and according to an Institutional Review Board approved protocol.
- Wipe the outside of the tubes with 70% ethanol. Carefully remove the tops of the filled collection tubes and decant the blood into a sterile 50 ml centrifuge tube.
- Add 10 ml sterile phosphate buffered saline (PBS) to each blood collection tube. Collect the PBS, add to the decanted whole blood, and mix gently.
- Add 25 µl Human T Cell Enrichment Cocktail / 2 ml total volume. Incubate at room temperature for 20 min.
- Using a 10 ml pipette, layer up to 10 ml of the 1:1 diluted blood gently on top of the density gradient medium. Be careful to not disrupt the surface of the density gradient medium.
- Centrifuge layered blood and density gradient medium at 1,200 x g for 20 min at 20 oC.
- Remove the tubes from the centrifuge, taking care to not disrupt the interface between the density gradient medium and the layer of leukocytes at the interface between the density gradient medium and the diluted plasma. Using a 5 ml pipette, carefully collect the leukocyte layer and transfer to a new sterile 50 ml conical tube.
- Add PBS to bring the volume of the collected PBL to 50 ml and mix gently.
- Centrifuge at 600 x g for 5 min at 20 oC. Decant.
- Resuspend pelleted cells in 10 ml sterile flow cytometry sorting buffer (sterile-filtered PBS containing 1% BSA). Sample 10 µl of cell suspension, dilute 1:10 (add to 90 μl) trypan blue to count cells using a hemocytometer (expected yield 1 – 4 x 106 cells / tube of whole blood).
- Remove 1 ml aliquot, transfer to 5 ml tube, and keep on ice for analysis of T cells not labeled by tetramer. Keep cell suspension on ice.
2. Magnetic Enrichment of Alloantigen-specific T cells
- Dilute alloantigen pMHC tetramer to 1:100 in sterile sort buffer.
- Centrifuge cell suspension (9 ml from step 1.11) at 600 x g for 5 min at 20 oC. Decant.
- Add 50 µl of diluted alloantigen pMHC tetramer to pelleted cells (up to 107 cells). Mix by gentle pipetting. Transfer to a sterile 5 ml tube. Incubate for 30 min at room temperature.
- Add 2 ml sort buffer. Centrifuge at 600 x g for 5 min at 20 oC. Decant.
- Resuspend cells in 100 µl sort buffer. Add 10 µl Biotin Selection Cocktail and incubate for 15 min at room temperature.
- Add 5 µl Magnetic Nanoparticles and incubate for 10 min at room temperature.
- Add 2.5 ml sort buffer and mix gently by pipetting. Remove 100 µl aliquot, transfer to 5 ml tube, and keep on ice for analysis of pre-enrichment cells.
- Place the 5 ml tube containing the cell suspension in the cell separation magnet. The cap should be loosely atop the tube. Incubate for 5 min at room temperature.
- Gently remove the cap from the tube. Holding the tube and magnet together, decant the tube contents into a fresh 5 ml tube. Do not tap or shake the tube contents to remove the last drops of liquid.
- Remove the tube from the magnet. Add 2 ml cold sort buffer and mix gently by pipetting. Sample 10 µl of cell suspension, dilute 1:2 (add to 10 µl) trypan blue to count cells using a hemocytometer.
3. Preparation of T cells for Single-cell Flow Cytometry Cell Sorting
- Add cold sort buffer to all tubes of cells (tetramer unlabeled, tetramer-labeled un-enriched, and tetramer-labeled enriched) to bring volume to 3 ml.
- Centrifuge at 600 x g for 5 min at 4 oC and decant buffer to recover cells.
- Resuspend pelleted cells in 25 µl cold sort buffer. Add 5 µl human Fc block. Incubate on ice for 20 min.
- Prepare antibodies for flow cytometry sorting. Mix 15 µl sort buffer, 15 µl anti-CD5, 15 µl anti-CD14, and 15 µl anti-CD19.
- Add 20 µl of antibody mixture to each tube of cells. Incubate on ice for 20 min.
- Add 2 ml of cold sort buffer to each tube.
- Centrifuge at 600 x g for 5 min at 4 oC and decant buffer to recover cells.
- Resuspend cells at a concentration of 1 -2 x 106 cells/ml in sort buffer.
- Aliquot 100 µl of human T cell culture medium (Iscove’s DMEM supplemented with 2 mM Glutamax, 10 mM HEPES, 50 µg/ml gentamycin, 50 µM 2-mercaptoethanol, 10% heat-inactivated human AB serum, and 2.5 ng/ml recombinant human IL-2) to each well of a 96-well round bottom cell culture plate. Keep on ice.
4. Isolation of Tetramer-labeled T cells by Single-cell Flow Cytometry Sorting
- Establish flow cytometry gating parameters to identify alloantigen-pMHC tetramer labeled T cells (Figure 2.A). Use the pre-enrichment tetramer labeled fraction to establish gating strategies to eliminate doublets, gate on lymphocytes, exclude CD19 expressing B cells and CD14 expressing monocytes, and identify T cells by CD5 expression. Use the sample not labeled with tetramer as a fluorescence minus one control to establish the gating for identification of tetramer-binding T cells (with 0% of CD5+CD14-CD19- cells from the sample not labeled by tetramer should fall within this gate).
NOTE: Gating strategies that are not adequately stringent will result in isolation of non-T cells or T cells that are not antigen specific, while overly restrictive gating strategies will reduce the number of T cells isolated. - Program the plate parameters for the single cell sort. Direct the sorter to place 1 CD5+CD14-CD19-tetramer+ cell in each well. Direct the plate set up to contain 1 negative control well (no cells sorted into the well) and 1 positive control well (100 CD5+CD14-CD19-tetramer- cells) in each row (Figure 2.B).
- Using the established flow cytometry gating strategy and plate setup, isolate individual tetramer-binding T cells directly into the 96-well plate using a flow cytometric cell sorter.
5. Culture and Expansion of Alloantigen-specific T cell Clones
- After completion of cell sorting, centrifuge plate at 600 x g for 5 min at 20 oC. Place culture plate at 37 oC in a 6% CO2 incubator.
- Vortex stimulatory anti-CD3/anti-CD28 microbeads for 30 sec to resuspend beads.
- Calculate volume of activator beads required for stimulation of collected T cells (0.5 µl of activator microbeads / well). Transfer calculated volume of stimulator beads to a sterile 5 ml tube. Add 1 ml human T cell culture medium and vortex.
- Place tube with microbead suspension in magnet. The cap should be loosely atop the tube. Incubate for 2 min at room temperature.
- Gently remove the cap from the tube. Holding the tube and magnet together, decant tube contents into a fresh 5 ml tube. Do not tap or shake the tube contents.
- Remove the tube from the magnet. Add human T cell culture medium (100 µl medium / 0.5 µl of microbeads initially added).
- Aliquot 100 µl of activator bead suspension to each well of the 96-well plate. Incubate culture plate at 37 oC in a 6% CO2 incubator.
- Monitor cell growth daily by microscopic examination. Small clusters of proliferating cells may be observed microscopically after 5 – 7 days of culture (Figure 3.A).
NOTE: Visualization of T cell cultures at this point can be difficult, and the absence of observable cell clusters at this stage may not be indicative of a lack of growing T cells. - At 14 days after cell isolation, feed cultures by carefully removing 100 µl of media off of the top of the culture and replace with 100 µl fresh human T cell culture medium. Continue incubating culture plate at 37 oC in a 6% CO2 incubator.
- Monitor cell growth daily by microscopic examination and evaluation of the color of the culture medium. Large cell clusters should be visible microscopically 2 – 3 days after the medium change. Macroscopically, cell pellets should become visible during the 14 days following the medium change.
- At 28 days following cell isolation, identify growing clones by microscopic examination. Transfer the 200 µl volume of the growth-positive 96-well plate cultures to individual wells of a 48-well tissue culture plate containing 200 µl human T cell culture medium.
- Add 100 µl containing 12.5 µl of stimulatory anti-CD3/anti-CD28 microbeads (prepared as described in sections 5.2 – 5.6). Incubate culture plate at 37 oC in a 6% CO2 incubator.
- Monitor culture growth daily by microscopic examination and evaluation of the color of the culture medium. When T cell culture reaches >2x106/ml (typically 3-5 days after re-stimulation, can be estimated by the day that the media begins turning straw-yellow), transfer culture to a well of a 24-well tissue culture plate and add 500 µl human T cell medium.
- At 10-14 days follow re-stimulation, collect 200 µl of T cell culture to assess alloantigen specificity by flow cytometry analysis of pMHC tetramer binding (using the labeling and gating strategy described in sections 3 and 4.1).
6. Long-term Re-stimulation and Culture of T cell Clones
- Continually re-stimulate and expand clonal T cell cultures with the desired specificity can be every 14 days. Collect T cell cultures into a sterile 5 ml tube at day 14 following the last anti-CD3/CD28 microbead stimulation. Combine multiple wells of the same T cell clone in a single tube, up to a volume of 2.5 ml.
- Add medium to bring the final volume to 2.5 ml and mix gently by pipetting.
- Place the 5 ml tube containing the T cell culture into the cell separation magnet to remove old microbeads from the culture. The cap should be loosely atop the tube. Incubate for 5 min at room temperature.
- Gently remove the cap from the tube. Holding the tube and magnet together, decant the cell suspension into a fresh 5 ml tube. Do not tap or shake the tube contents to remove the last drops of liquid.
- Add medium to bring the final volume to 4 ml. Sample 10 µl to count cells using a hemocytometer.
- Recover T cells by centrifugation at 600 x g for 5 min at room temperature.
- Decant supernatant. Resuspend T cells at 106 cells/ml human T cell culture medium. Transfer 1 ml aliquots to each well of a 24-well tissue culture plate.
- Re-stimulate T cells by adding 100 µl media containing 12.5 µl of stimulatory anti-CD3/anti-CD28 microbeads (as described in sections 5.2 – 5.6). Incubate at 37 oC in a 6% CO2 incubator.
Wyniki
This protocol describes the generation of clonal human T cell cultures with defined alloantigen specificity via a magnetic bead enrichment and single-cell flow cytometry sorting strategy. Figure 1 provides an outline of the process.
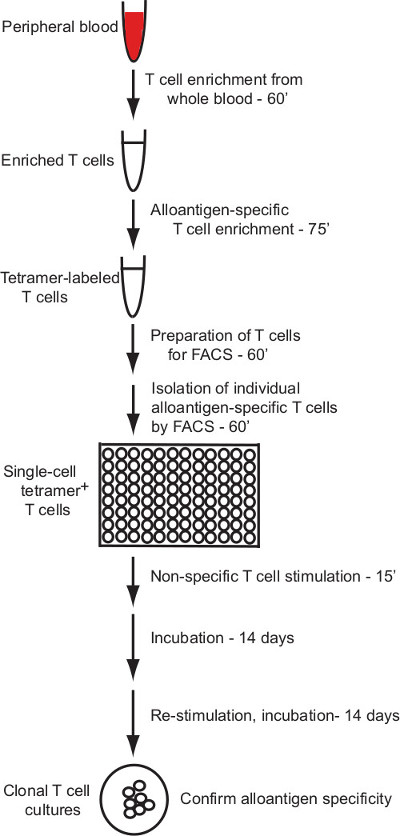
Figure 1: Protocol overview. The protocol described here provides a reliable method for generation of alloantigen...
Dyskusje
T cell alloreactivity is a long-studied and clinically-relevant phenomenon. The robust proliferative and effector responses of T cells to allogeneic stimulation has enabled extensive analyses of human T cell responses in vitro through relatively straightforward mixed lymphocyte reactions of peripheral blood T cells against inactivated allogeneic cells. However, these primary alloreactive T cell responses are oligoclonal, comprised of a large number of individual T cells responding to specific alloantigens. This ...
Ujawnienia
The authors declare no competing financial interests.
Podziękowania
The author would like to thank the NIH Tetramer Core Facility for tetramer production. The author would also like to thank E.D. O’Connor and K.E. Marquez at the UCSD Human Embryonic Stem Cell Core Facility flow cytometry laboratory for assistance in cell sorting. This work was funded by National Institutes of Health grant K08AI085039 (G.P.M.).
Materiały
| Name | Company | Catalog Number | Comments |
| Sodium heparin venous blood collection tube 16 x 100 mm | Becton, Dickenson and Company | 366480 | |
| Lymphoprep | Stemcell Technologies | 7801 | |
| Rosette Sep Human T Cell Enrichment Kit | Stemcell Technologies | 15061 | |
| Dulbecco's PBS, 1x without Ca or Mg | Corning | 21-031-CV | |
| Bovine serum albumin | Sigma-Aldrich | A7906 | |
| EDTA | Sigma-Aldrich | E6635 | |
| Fluorophore-labeled pMHC tetramer | NIH Tetramer Facility | NA | |
| EasySep Biotin Selection Kit | Stemcell Technologies | 18553 | |
| EasySep Selection magnet | Stemcell Technologies | 18000 | |
| TruStain FcX Human Fc blocking solution | Biolegend | 422301 | |
| Anti-CD5 PE-Cy7 (clone UCHT2) | Biolegend | 300621 | |
| Anti-CD14 FITC (clone HCD14) | Biolegend | 325603 | |
| Anti-CD19 FITC (clone HIB19) | Biolegend | 302205 | |
| Iscove's DMEM, without b-ME or L-glutamine | Corning | 15-016-CV | |
| HEPES | Corning | 25-060-CI | |
| b-Mercaptoethanol | Life Technologies | 21985-023 | |
| Glutamax | Life Technologies | 35050061 | |
| Gentamicin sulfate (50 mg/ml) | Omega Scientific | GT-50 | |
| Human AB serum, male donor | Omega Scientific | HS-30 | |
| Recombinant human IL-2 | Peprotech | AF 200-02 | |
| Dynabeads Human T-Activator CD3/CD28 | Life Technologies | 11131D | |
| Media | |||
| Cell sorting buffer | |||
| PBS, pH 7.4 | 1 L | ||
| BSA | 10 g | ||
| EDTA (0.5 M) | 2 ml | ||
| Human T Cell Culture Medium | |||
| Iscove's DMEM | 351.6 ml | ||
| Heat-inactivated human AB serum | 40 ml | ||
| HEPES (1 M) | 4 ml | ||
| Glutamax (100x) | 4 ml | ||
| Gentamicin (50 mg/ml) | 0.4 ml | ||
| b-mercaptoethanol (14.3 M) | 1.4 ml | ||
| Recombinant human IL-2 (1 mg/ml) | 1 ml |
Odniesienia
- Smith-Garvin, J. E., Koretzky, G. A., Jordan, M. S. T cell activation. Annu. Rev. Immunol. 27 (1), 591-619 (2009).
- Morris, G. P., Allen, P. M. How the TCR balances sensitivity and specificity for the recognition of self and pathogens. Nat. Immunol. 13 (2), 121-128 (2012).
- Arstilla, T. P., et al. A direct estimation of the human αβ T cell receptor diversity. Science. 286 (5441), 958-961 (1999).
- Robbins, H. S., et al. Comprehensive assessment of T-cell receptor β-chain diversity in αβ T cells. Blood. 114 (19), 4099-4107 (2009).
- Alanio, C., Lemaitre, F., Law, H. K. W., Hasan, M., Albert, M. L. Enumeration of human antigen-specific naive CD8+ T cells reveals conserved precursor frequencies. Blood. 115 (18), 3718-3725 (2010).
- Suchin, E. J., et al. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J. Immunol. 166 (2), 973-981 (2001).
- Felix, N. J., Allen, P. M. Specificity of T-cell alloreactivity. Nat. Rev. Immunol. 7 (12), 942-953 (2007).
- Morris, G. P., Ni, P. P., Allen, P. M. Alloreactivity is limited by the endogenous peptide repertoire. Proc. Natl. Acad. Sci. USA. 108 (9), 3695-3700 (2011).
- Suri, A., et al. In APCs, the autologous peptides selected by the diabetogenic I-Ag7 molecule are unique and determined by the amino acid changes in the P9 pocket. J. Immunol. 168 (3), 1235-1243 (2002).
- Yssl, H., Spits, H. Generation and maintenance of cloned human T cell lines. Curr. Protoc. Immunol. 7, (2002).
- Altman, J. D., et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 274 (5284), 94-96 (1996).
- Moon, J. J., et al. Naive CD4+ T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 27 (2), 203-213 (2007).
- Chicz, R. M., et al. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J. Exp. Med. 178 (1), 27-47 (1993).
- Ni, P. P., Allen, P. M., Morris, G. P. The ability to rearrange dual TCRs enhances positive selection, leading to increased allo- and autoreactive T cell repertoires. J. Immunol. In press, (2014).
- Morris, G. P., Uy, G. L., Donermeyer, D., DiPersio, J. F., Allen, P. M. Dual receptor T cells mediate pathologic alloreactivity in patients with acute graft-versus-host disease. Sci. Transl. Med. 5 (188), (2013).
- Altman, J. D., Reay, P. A., Davis, M. M. Formation of functional peptide complexes of class II major histocompatibility complex proteins from subunits produced in Escherichia coli. Proc. Natl. Acad. Sci. USA. 90 (21), 10330-10334 (1993).
- Sabatino, J. J., Huang, J., Zhu, C., Evavold, B. D. High prevalence of low affinity peptide-MHC II tetramer-negative effectors during polyclonal CD4+ T cell responses. J. Exp. Med. 208 (1), 81-90 (2011).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone