Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Simultaneous Detection of c-Fos Activation from Mesolimbic and Mesocortical Dopamine Reward Sites Following Naive Sugar and Fat Ingestion in Rats
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
The goal of this study is to identify reward-related distributed brain networks by delineating a reliable immunohistological technique using cellular c-fos activation to measure simultaneous changes in dopamine pathways and terminal sites after novel ingestion of fat and sugar in rats.
Streszczenie
This study uses cellular c-fos activation to assess effects of novel ingestion of fat and sugar on brain dopamine (DA) pathways in rats. Intakes of sugars and fats are mediated by their innate attractions as well as learned preferences. Brain dopamine, especially meso-limbic and meso-cortical projections from the ventral tegmental area (VTA), has been implicated in both of these unlearned and learned responses. The concept of distributed brain networks, wherein several sites and transmitter/peptide systems interact, has been proposed to mediate palatable food intake, but there is limited evidence empirically demonstrating such actions. Thus, sugar intake elicits DA release and increases c-fos-like immunoreactivity (FLI) from individual VTA DA projection zones including the nucleus accumbens (NAC), amygdala (AMY) and medial prefrontal cortex (mPFC) as well as the dorsal striatum. Further, central administration of selective DA receptor antagonists into these sites differentially reduce acquisition and expression of conditioned flavor preferences elicited by sugars or fats. One approach by which to determine whether these sites interacted as a distributed brain network in response to sugar or fat intake would be to simultaneous evaluate whether the VTA and its major mesotelencephalic DA projection zones (prelimbic and infralimbic mPFC, core and shell of the NAc, basolateral and central-cortico-medial AMY) as well as the dorsal striatum would display coordinated and simultaneous FLI activation after oral, unconditioned intake of corn oil (3.5%), glucose (8%), fructose (8%) and saccharin (0.2%) solutions. This approach is a successful first step in identifying the feasibility of using cellular c-fos activation simultaneously across relevant brain sites to study reward-related learning in ingestion of palatable food in rodents.
Wprowadzenie
Brain dopamine (DA) has been implicated in central responses to intake of palatable sugars through proposed hedonic1,2, effort-related3 and habit-based4,5 mechanisms of action. The primary DA pathway implicated in these effects originates in the ventral tegmental area (VTA), and projects to the nucleus accumbens (NAC) core and shell, the basolateral and central-cortico-medial amygdala (AMY), and the prelimbic and infralimbic medial prefrontal cortex (mPFC) (see reviews6,7). The VTA has been implicated in sucrose intake8,9, and DA release is observed following sugar intake in the NAC10-15, AMY16,17 and mPFC18-20. Fat intake also stimulates DA NAC release21, and another DA-rich projection zone to the dorsal striatum (caudate-putamen) has been also associated with DA-mediated feeding22,23. Kelley24-27 proposed that these multiple projection zones of this DA-mediated system formed an integrated and interactive distributed brain network through extensive and intimate interconnections28-34.
In addition to the ability of DA D1 and D2 receptor antagonists to reduce intake of sugars35-37 and fats38-40, DA signaling has also been implicated in mediating the ability of sugars and fats to produce conditioned flavor preferences (CFP)41-46. Microinjections of a DA D1 receptor antagonist into the NAC, AMY or mPFC47-49 eliminate acquisition of CFP elicited by intragastric glucose. Whereas microinjections of either DA D1 or D2 receptor antagonists into the mPFC eliminates acquisition of fructose-CFP50, the acquisition and expression of fructose-CFP are differentially blocked by DA antagonists in the NAC and AMY51,52.
The c-fos technique53,54 has been employed to investigate neural activation induced by palatable intake and neural activation. The term "c-fos activation" will be used throughout the manuscript, and is operationally defined by increased transcription of c-Fos during neuronal depolarization. Sucrose intake increased fos-like immunoreactivity (FLI) in the central AMY nucleus, the VTA as well as the shell, but not core, of the NAC55-57. Whereas sucrose intake in sham-feeding rats significantly increased FLI in the AMY and the NAC, but not the VTA58, intragastric sucrose or glucose infusions significantly increased FLI in the NAC and central and basolateral nuclei of the AMY59,60. Repeated addition of sucrose to scheduled chow access increased FLI in the mPFC as well as the NAC shell and core61. A sucrose concentration downshift paradigm revealed that the greatest FLI increases occurred in the basolateral AMY and NAC, but not the VTA62. Following conditioning, extinction of sugar-related natural reward behaviors increased FLI in the basolateral AMY and the NAC63. Moreover, pairing sugar availability to a tone resulted in the tone subsequently increasing FLI levels in the basolateral AMY64. High-fat intake also increased FLI in NAC and mPFC sites65-67.
Most of the previously cited studies examined sugar and fat effects on c-fos activation in single sites that do not provide information about identification of reward-related distributed brain networks24-27. Further, many of the studies also did not delineate the relative contributions of sub-areas of the NAC (core and shell), AMY (basolateral and central-cortico-medial) and mPFC (prelimbic and infralimbic) that could potentially be examined by the advantage of excellent spatial, single-cell resolution in c-Fos mapping68. Our laboratory69 recently used c-fos activation and simultaneously measured alterations in the VTA DA pathway and its projection zones (NAC, AMY and mPFC) after novel ingestion of fats and sugars in rats. The present study describes the procedural and methodological steps to simultaneously analyze whether acute exposure to six different solutions (corn oil, glucose, fructose, saccharin, water and a fat emulsion control) would differentially activate FLI in sub-areas of the NAC, AMY, mPFC as well as the dorsal striatum. This simultaneous detection of differences allowed confirmation of significant effects on FLI in each site and determination as to whether changes in one particular site correlated with changes in related sites, thereby providing support for a distributed brain network24-27. These procedures tested whether the VTA, the prelimbic and infralimbic mPFC, the core and shell of the NAC, and the basolateral and central-cortico-medial AMY) as well as the dorsal striatum would display coordinated and simultaneous FLI activation after oral, unconditioned intake of glucose (8%), fructose (8%), corn oil (3.5%) and saccharin (0.2%) solutions.
Protokół
These experimental protocols have been approved by the Institutional Animal Care and Use Committee certifying that all subjects and procedures are in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
1. Subjects
- Purchase and/or breed male Sprague-Dawley rats (260 - 300 g).
- House rats individually in wire mesh cages. Maintain them on a 12:12 hr light/dark cycle with rat chow and water available ad libitum.
- Assign appropriate sample sizes (e.g., n ≈ 6 - 8) randomly into groups.
2. Testing Apparatus and Intake Procedures
- Use calibrated centrifuge tubes with rubber stoppers and a 45° angle metal sipper tube to provide accurate measurement (± 0.1 ml) of the presented solutions. Secure them to the home cages by a taut metal spring to allow visibility of the calibrations.
- Restrict food rations (~ 15/g/day) of the rats to reduce weight to 85% of their original body weight to increase motivation to consume the solutions. Note: The weight reduction should take between 3 - 5 days.
- Provide pre-training solutions (10 ml) of 0.2% saccharin for four days over a 1 hr session to maximize the probability that rats will sample the subsequent test solutions with short (less than 1 min) latency.
- Confirm flow through the centrifuge tube by spilling a few drops.
- Weigh tubes before and after each session to obtain intake measurement.
- Perform an intake test on the fifth day on subgroups receiving one of six solutions (10 ml, 1 hr): a) water, b) novel flavored (0.05% cherry flavor) 0.2% saccharin, c) 8% fructose, d) 8% glucose, e) 3.5% corn oil suspended in 0.3% xanthan gum, and f) 0.3% xanthan gum.
- Ensure that the nutrient solutions are isocaloric; thus, the 3.5% corn-oil concentration is isocaloric to the 8% sugar solutions.
- Ensure that rats sample solutions with short latency (less than 1 min). If this requirement is not met, then discard the subject from the study.
3. Tissue Preparation
- Anesthetize each animal by an intraperitoneal injection of pentobarbital 90 min after initial exposure to each test solution. Confirm that animals are properly anesthetized by demonstrating that the animal is no longer responsive to such reflexes as withdrawal to foot pinch, blinking following direct corneal pressure or head shaking to deep pinna stimulation.
- Perfuse each animal transcardially as described previously69.
- Anesthetize rats with an overdose of sodium pentobarbital (65 mg/kg), remove the ribcage and expose the chest for free access to the heart69.
- Place the needle in the apex of the left heart valve, and cut the vena cava. Administer phosphate buffer solution (PBS, ~ 180 ml) followed by a phosphate-buffered fixative containing 4% paraformaldehyde (~ 180 ml).
- Ensure that the animal actually is being perfused correctly by examining whether liquid is leaving other cavities, such as the nose, mouth, and genital areas. Note: Proper fixation with paraformaldehyde will be accompanied by large muscle movements. If this does not occur, re-adjust the needle until this reaction occurs.
- Remove the brain from the skull quickly by cutting fur and skin away from the skull. Use rongeurs to crack and remove the bone from the brain moving from rear to front. Work initially in the area below and behind the cerebellum, ensuring that the rongeur is between the bone and meningeal pia mater. Once the top and sides of the skull are removed, use a small spatula to lift the brain from the base, and snip cranial nerves with small scissors. Be careful not to damage the brain while attempting to remove the bone.
- Fix the brains in a 4% paraformaldehyde solution overnight at 4 °C. Place the brains in a 30% sucrose/70% PBS solution at room temperature until they settle at the bottom of the container.
- Block the brain
- Remove the rostral part of the brain cutting transversely caudal to the olfactory bulb.
- Remove the caudal part of the brain cutting transversely at the level of the cerebellum and the pons.
- Mount the brain coronally with the caudal part fixed to the stage of a sliding microtome, and cut coronal sections (40 µm) through the mPFC (+2.86 – +2.20 mm rostral to bregma), the NAC core and shell and dorsal striatum (+1.76 – +1.60 mm rostral to bregma), the AMY (-2.12 – -2.92 mm caudal to bregma), and the VTA (-5.20 – -5.60 mm caudal to bregma). Use a rat brain atlas70 for guidance.
- Collect free-floating sections into individual wells of a 24-well plate filled with PBS for eventual immunohistochemical analysis71. Use Parafilm to seal the 24 well plate to make sure the PBS does not evaporate in the container and dry up the brain. Store the brain tissue in 4 °C.
4. c-fos Procedures (Adapted from 71)
- Treat each section with 5 ml of 5% Normal Goat Serum and 0.2% Triton x-100 in PBS for 1 hr.
- Incubate the treated sections with primary antibodies (rabbit anti-c-fos, 1:5,000) at 4 °C for 36 hr in wells containing 1 ml of PBS.
- Rinse sections 3x with PBS (5 ml) for 10 min each.
- Incubate with secondary antibodies (biotinylated goat anti-rabbit; 1:200) at RT for 2 hr in wells containing 1 ml of PBS.
- Rinse each section 3x in PBS (5 ml) for 10 min each.
- Incubate the rinsed sections for 2 hr in a commercially available avidin-horseradish peroxidase mixture that comes in a kit composed of Avadin DH (100 µl) and Biotinylated Horseradish Peroxidase H (100 µl) in 5 ml of PBS.
- Re-rinse the sections 3x in PBS (5 ml) for 10 min each.
- React the sections with 0.05% diaminobenzidine (DAB) in the presence of 0.0015% H2O2 for 5 - 10 min, depending on the reactivity of the tissue in wells containing 5 ml of the DAB solution.
- Double-label the VTA sections. Incubate them with a tyrosine hydroxylase (TH) antibody (rabbit anti-rat TH, 1:2,000) in PBS (5 ml) overnight at 4 °C.
- Rinse sections 3x in PBS (5 ml) for 10 min each.
- Incubate with secondary antibodies (biotinylated goat anti-rabbit; 1:200) in PBS (5 ml) at room temperature for 2 hr.
- Rinse sections 3x in PBS (5 ml) for 10 min each.
- Visualize the antibodies by using a secondary antibody-peroxidase complex. React with a combination of 0.05% DAB and a 0.3% nickel sulfate solution, for 5 - 10 min, depending on the reaction of the tissue in wells containing 5 ml of the DAB/NiCl solution.
- Ensure that the DAB solution is milky light green in color from its reaction with the 0.3% nickel sulfate. If the solution is too green, then the reaction will be too dark.
- Mount all sections onto gelatin-coated slides. Let them dry overnight, and then cover-slip with a few drops of a Toluene-Based Solution (TBS).
- Code slides so that the experimental condition is unknown to the observers.
5. Determination of c-fos Immunoreactive Counts
- Assign pairs of unbiased observers to count Fos-positive neurons in these regions of interest (ROI): prelimbic mPFC, infralimbic mPFC, NAC core, NAC shell, basolateral AMY nuclei, central-cortico-medial AMY, dorsal striatum, and VTA. Delineate whether c-Fos immunoreactivity was present in TH+ and TH- cells in the VTA. Figure 1 provides a screen-captured picture of the NAC from the microscope.
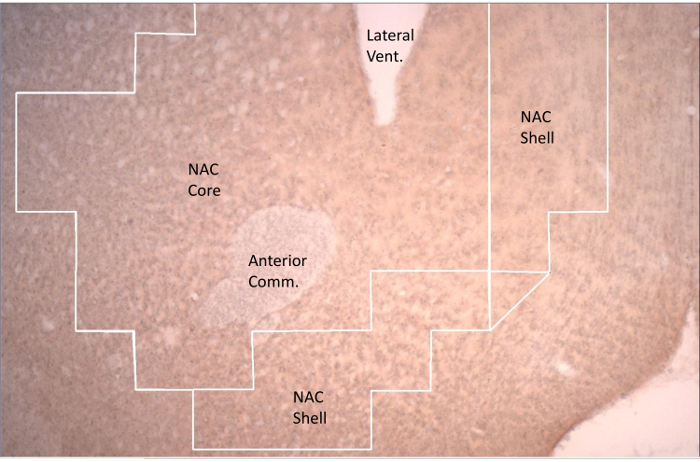
Figure 1. Representative Section of the Nucleus Accumbens (NAC) Displaying Regions of Interest Outlined by the Grid by which c-fos Counts are Made. The NAC shell is found medially and ventrally to the NAC core. The NAC core encircles the anterior commissure (Anterior Comm.). The ventral extent of the lateral ventricle (Lateral Vent.) is visible. Please click here to view a larger version of this figure.
- Analyze at least three representative slices per site common to all animals in all of the testing conditions.
- Use software and an optical microscope to analyze the entire region for each ROI by tracing an outline (Figure 1).
- For a given site, open the application and click on the acquisition drop-down menu and click "Live Image". Bring the ROI into focus and click the screen to establish a reference point. Then trace the chosen brain region using the grid as a guide. Once the trace is completed, count cells (steps 5.3.1.1 - 5.3.1.3).
- Double-click the software icon. Go to the menu bar, click on "Acquisition" and then "Live Image". Bring the ROI into focus and click the screen to establish a reference point.
- Go to the grid toolbar and click "Display Grid" and "Use grid labels". Outline the ROI with a predetermined trace.
- Count all cells in each ROI area, select a "+" in the left hand sidebar to keep count of c-fos cells. Click each cell singly to register counts. Consider a cell positive for c-fos when a defined dark red circle is observed (Figure 1).
- Repeat this process for each site.
- Record counts in a lab notebook and on the computer for future analysis. Go to the menu bar, click on "File", "Save Data File" to save the tracing and counts.
- For a given site, open the application and click on the acquisition drop-down menu and click "Live Image". Bring the ROI into focus and click the screen to establish a reference point. Then trace the chosen brain region using the grid as a guide. Once the trace is completed, count cells (steps 5.3.1.1 - 5.3.1.3).
- Ensure that inter-rater reliability (using correlation of counts) of the two uninformed raters for each section in each ROI always exceeds 0.8.
6. Statistics
- Evaluate baseline saccharin intakes over the first four days using a repeated-measures 1-way analysis of variance (ANOVA) comparing the saccharin intakes of Days 1, 2, 3 and 469.
- Compare saccharin intake (Day 4) with test intakes (Day 5) of the six groups using a randomized-block 2-way ANOVA69.
- Use Tukey comparisons (p < 0.05) to determine individual significant effects69.
- Determine inter-rater reliability, and then use a common observer's counts.
- Average c-fos counts for the three representative slices for each site69.
- Perform a 1-way ANOVA of c-fos activation induced by intake of the six solutions (3.5% corn oil, 8% glucose, 8% fructose, 0.2% flavored saccharin, xanthan gum control and water) for the perilimbic mPFC69.
- Repeat parallel analyses of the six groups for infralimbic mPFC, NAC core, NAC shell, basolateral AMY, central-cortico-medial AMY, VTA and dorsal striatum. Use Tukey comparisons (p < 0.05) to reveal individual significant effects69.
- Compare corn oil intake with both water intake and intake of its suspension agent, xanthan gum. Compare fructose and glucose intakes with both water intake and intake of the non-nutritive sweetener, saccharin.
- Establish whether significant relationships between solution intakes and c-fos activation in each of the sites were observed using Bonferroni r correlations (p < 0.05).
- Systematically compare c-fos counts in the perilimbic and infralimbic pre-frontal cortex for each animal in the 3.5% corn oil group.
- Repeat systematically parallel analyses of each pair of the six sites (VTA, dorsal striatum, infralimbic mPFC, perilimbic mPFC, NAC core, NAC shell, basolateral AMY, central-cortico-medial AMY) for the 3.5% corn oil.
- Repeat systematically parallel analyses of these six sites for the other five experimental intake conditions (8% glucose, 8% fructose, 0.2% flavored saccharin, xanthan gum control and water).
- Take advantage of the fact that the same animals within a solution condition were evaluated across all sites by determining significant relationships between c-fos activation across solutions and within each solution using Bonferroni r correlations (p < 0.05).
Wyniki
All representative results described below have been published previously69, and are re-presented here to support "proof of concept" in indicating the effectiveness of the technique.
Solution Intakes
Significant differences in baseline saccharin intakes were observed over the first four days for all animals (F(3,108) = 57.27, p < 0.001) with intakes (Day 1: 1.3 (± 0.2) ml; Day 2...
Dyskusje
The goal of the study was to determine if the source (VTA) and forebrain projection targets (NAC, AMY, mPFC) of DA reward-related neurons were simultaneously activated after novel ingestion of fat and sugar in rats using the cellular c-fos technique. The present study is a detailed description of the protocols of a study published previously69. It was hypothesized that the VTA, its major projection zones to the prelimbic and infralimbic mPFC, the core and shell of the NAC and the basolateral and central-cortic...
Ujawnienia
The authors have no competing financial interests.
Podziękowania
Thanks to Diana Icaza-Culaki, Cristal Sampson and Theologia Karagiorgis for their hard work on this project.
Materiały
| Name | Company | Catalog Number | Comments |
| Equipment | |||
| Sprague-Dawley rats | Charles River Laboratories | CD-1 | |
| Wire Mesh Cages | Lab Products, Seaford, DE | 30-Cage rack | |
| Rat Chow | PMI Nutrition International | 5001 | |
| Taut Metal Spring | Lab Products, Seaford, DE | n/a | |
| Rat Weighing Scale | Fisher Scientific Company | n/a | |
| Nalgene Centrifuge Tubes | Lab Products, Seaford, DE | 10-0501 | |
| Rubber Stopper | Lab Products, Seaford, DE | n/a | |
| Metal Sippers | Lab Products, Seaford, DE | n/a | |
| Saccharin | Sigma Chemical Co | 82385-42-0 | |
| Kool-Aid, Cherry | Kool-Aid | Commerical | |
| Kool-Aid, Grape | Kool-Aid | Commercial | |
| Fructose | Sigma Chemical Co | F0127 | |
| Glucose | Sigma Chemical Co | G8270 | |
| Corn Oil | Mazzola | Commerical | |
| Xanthan Gum | Sigma Chemical Co | 11138-66-2 | |
| Sliding Microtome | Microm International | n/a | |
| Neurolucida Camera | MBF Bioscience | Software application | |
| Gelatin-coated Slides | Fisher Scientific Company | 12-550-343 | |
| Cover glass | Fisher Scientific Company | 12-545-M | |
| Golden Nylon Brushes | Loew-Cornell | 2037 | |
| Natural Hair Sable | Loew-Cornell | 2022 | |
| 24 Well Plates | Fisher Scientific | 3527 | |
| 6 Well Plates | Fisher Scientific | 3506 | |
| 1 L Pyrex bottles | Fisher Scientific | 1395-1L | |
| Tissue insert (tissue strainer) | Fisher Scientific | 7200214 | |
| Eagle pipettes | World Precision Instruments | E10 for 1-10μl | |
| Eagle pipettes | World Precision Instruments | E100 for 20-100μl | |
| Eagle pipettes | World Precision Instruments | E200 for 50-200μl | |
| Eagle pipettes | World Precision Instruments | E1000 for 100-1000μl | |
| Eagle pipettes | World Precision Instruments | E5000 for 1000-5000μl | |
| Universal Tips .1-10 μl | World Precision Instruments | 500192 | |
| Universal Tips 5-200 μl | World Precision Instruments | 500194 | |
| Universal Tips 500-5,000 μl | World Precision Instruments | 500198 | |
| Blade Vibroslice 100 | World Precision Instruments | BLADE | |
| DPX Mounting Medium | Electron Microscopy | 13510 | |
| 15 ml centrifuge tubes | Biologix Research Co. | 10-0501 | |
| Slide Boxes | Biologix Research Co. | 41-6100 | |
| Orbital Shaker | Madell Corporation | ZD-9556 | |
| weigh boats | Fisher Scientific | 02-202-100 | |
| 5 ml disposable pipettes | Fisher Scientific | 13-711-5AM | |
| Stereo Investigator Software | Micro Bright Field | Software application | |
| Name | Company | Catalog number | Comments |
| Reagents | |||
| Paraformaldehyde Granular | Fisher Scientific | 19210 | |
| NaCl | Fisher Scientific | S271-1 | |
| Sodium Phophate Monobasic | Fisher Scientific | S468-500 | |
| Sodium Phosphate Diphasic | Fisher Scientific | BP332-500 | |
| Hydrogen Peroxide | Fisher Scientific | H324-500 | |
| SafeClear II | Fisher Scientific | 23-044-192 | |
| Methanol | Fisher Scientific | A412-1 | |
| Normal Goat Serum | Vector | S-1000 | |
| Biotinylated Anti-Rabbit IgG (H+L) | Vector | BA-1000 | |
| ABC Kit Peroxidase Standard | Vector | PK-4000 | |
| Anti-cFos (Ab-5) Rabbit | EMD chem/Cal Biochem | PC38 | |
| Triton X 100 | SigmaAldrich | X-100 | |
| 3,3' diaminobenzidine tetra hydrochloride | SigmaAldrich | D5905 | |
| Sodium Hydroxide | SigmaAldrich | 5881 | |
| Primary TH anti body | EMD Millipore | AB152 | |
| Euthosol | Virbac AH |
Odniesienia
- Koob, G. F. Neural mechanisms of drug reinforcement. Ann. N.Y. Acad. Sci. 654, 171-191 (1992).
- Wise, R. A. Role of brain dopamine in food reward. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 361, 1149-1158 (2006).
- Salamone, J. D., Correa, M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 76, 470-485 (2012).
- Horvitz, J. C., Choi, W. Y., Morvan, C., Eyny, Y., Balsam, P. D. A "good parent" function of dopamine: transient modulation of learning and performance during early stages of training. Ann. N.Y. Acad. Sci. 1104, 270-288 (2007).
- Wickens, J. R., Horvitz, J. C., Costa, R. M., Killcross, S. Dopaminergic mechanisms in actions and habits. J. Neurosci. 27, 8181-8183 (2007).
- Bjorklund, A., Dunnett, S. B. Dopamine neuron systems in the brain: an update. Trends Neurosci. 30, 194-202 (2007).
- Swanson, L. W. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res. Bull. 9, 321-353 (1982).
- Cacciapaglia, F., Wrightman, R. M., Careli, R. M. Rapid dopamine signaling differentially modulates distinct microcircuits within the nucleus accumbens during sucrose-directed behavior. J. Neurosci. 31, 13860-13869 (2011).
- Martinez-Hernandez, J., Lanuza, E., Martinez-Garcia, F. Selective dopaminergic lesions of the ventral tegmental area impair preference for sucrose but not for male sex pheromones in female mice. Eur. J. Neurosci. 24, 885-893 (2006).
- Bassareo, V., Di Chiara, G. Differential influence of associative and nonassociative learning mechanisms on the responsiveness of prefrontal and accumbal dopamine transmission to food stimuli in rats fed ad libitum. J. Neurosci. 17, 851-861 (1997).
- Bassareo, V., Di Chiara, G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neurosci. 89, 637-641 (1999).
- Cheng, J., Feenstra, M. G. Individual differences in dopamine efflux in nucleus accumbens shell and core during instrumental conditioning. Learn. Mem. 13, 168-177 (2006).
- Genn, R. F., Ahn, S., Phillips, A. G. Attenuated dopamine efflux in the rat nucleus accumbens during successive negative contrast. Behav. Neurosci. 118, 869-873 (2004).
- Hajnal, A., Norgren, R. Accumbens dopamine mechanisms in sucrose intake. Brain Res. 904, 76-84 (2001).
- Hajnal, A., Smith, G. P., Norgren, R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am. J. Physiol. 286, R31-R37 (2003).
- Bassareo, V., Di Chiara, G. Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur. J. Neurosci. 11, 4389-4397 (1999).
- Hajnal, A., Lenard, L. Feeding-related dopamine in the amygdala of freely moving rats. Neuroreport. 8, 2817-2820 (1997).
- Bassareo, V., De Luca, M. A., Di Chiara, G. Differential expression of motivational stimulus properties by dopamine in nucleus accumbens shell versus core and prefrontal cortex. J. Neurosci. 22, 4709-4719 (2002).
- Feenstra, M., Botterblom, M. Rapid sampling of extracellular dopamine in the rat prefrontal cortex during food consumption, handling, and exposure to novelty. Brain Res. 742, 17-24 (1996).
- Hernandez, L., Hoebel, B. G. Feeding can enhance dopamine turnover in the prefrontal cortex. Brain Res. Bull. 25, 975-979 (1990).
- Liang, N. C., Hajnal, A., Norgren, R. Sham feeding corn oil increases accumbens dopamine in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R1236-R1239 (2006).
- Dunnett, S. B., Iversen, S. D. Regulatory impairments following selective kainic acid lesions of the neostriatum. Behav. Brain Res. 1, 497-506 (1980).
- Salamone, J. D., Zigmond, M. J., Stricker, E. M. Characterization of the impaired feeding behavior in rats given haloperidol or dopamine-depleting brain lesions. Neurosci. 39, 17-24 (1990).
- Kelley, A. E. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci. Biobehav. Rev. 27, 765-776 (2004).
- Kelley, A. E. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 44, 161-179 (2004).
- Kelley, A. E., Baldo, B. A., Pratt, W. E. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal and food reward. J. Comp. Neurol. 493, 72-85 (2005).
- Kelley, A. E., Baldo, B. A., Pratt, W. E., Will, M. J. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol. Behav. 86, 773-795 (2005).
- Berendse, H. W., Galis-de-Graaf, Y., Groenewegen, H. J. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J. Comp. Neurol. 316, 314-347 (1992).
- Brog, J. S., Salyapongse, A., Deutch, A. Y., Zahm, D. S. The patterns of afferent innervation of the core and shell in the "accumbens" part of rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J. Comp. Neurol. 338, 255-278 (1993).
- McDonald, A. J. Organization of amygdaloid projections to the prefrontal cortex and associated stritum in the rat. Neurosci. 44, 1-14 (1991).
- McGeorge, A. J., Faull, R. L. The organization of the projection from the cerebral cortex to the striatum in the rat. Neurosci. 29, 503-537 (1989).
- Sesack, S. R., Deutch, A. Y., Roth, R. H., Bunney, B. S. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J. Comp. Neurol. 290, 213-242 (1989).
- Wright, C. I., Beijer, A. V., Groenewegen, H. J. Basal amygdaloid complex afferents to the rat nucleus accumbens are compartmentally organized. J. Neurosci. 16, 1877-1893 (1996).
- Wright, C. I., Groenewegen, H. J. Patterns of convergence and segregation in the medial nucleus accumbens of the rat: relationships of prefrontal cortical, midline thalamic and basal amygdaloid afferents. J. Comp. Neurol. 361, 383-403 (1995).
- Geary, N., Smith, G. P. Pimozide decreases the positive reinforcing effect of sham fed sucrose in the rat. Pharmacol. Biochem. Behav. 22, 787-790 (1985).
- Muscat, R., Willner, P. Effects of selective dopamine receptor antagonists on sucrose consumption and preference. Psychopharmacol. 99, 98-102 (1989).
- Schneider, L. H., Gibbs, J., Smith, G. P. D-2 selective receptor antagonists suppress sucrose sham feeding in the rat. Brain Res. Bull. 17, 605-611 (1986).
- Baker, R. W., Osman, J., Bodnar, R. J. Differential actions of dopamine receptor antagonism in rats upon food intake elicited by mercaptoacetate or exposure to a palatable high-fat diet. Pharmacol. Biochem. Behav. 69, 201-208 (2001).
- Rao, R. E., Wojnicki, F. H., Coupland, J., Ghosh, S., Corwin, R. L. Baclofen, raclopride and naltrexone differentially reduce solid fat emulsion intake under limited access conditions. Pharmacol. Biochem. Behav. 89, 581-590 (2008).
- Weatherford, S. C., Smith, G. P., Melville, L. D. D-1 and D-2 receptor antagonists decrease corn oil sham feeding in rats. Physiol. Behav. 44, 569-572 (1988).
- Azzara, A. V., Bodnar, R. J., Delamater, A. R., Sclafani, A. D1 but not D2 dopamine receptor antagonism blocks the acquisition of a flavor preference conditioned by intragastric carbohydrate infusions. Pharmacol. Biochem. Behav. 68, 709-720 (2001).
- Baker, R. M., Shah, M. J., Sclafani, A., Bodnar, R. J. Dopamine D1 and D2 antagonists reduce the acquisition and expression of flavor-preferences conditioned by fructose in rats. Pharmacol. Biochem. Behav. 75, 55-65 (2003).
- Dela Cruz, J. A., Coke, T., Icaza-Cukali, D., Khalifa, N., Bodnar, R. J. Roles of NMDA and dopamine D1 and D2 receptors in the acquisition and expression of flavor preferences conditioned by oral glucose in rats. Neurobiol. Learn. Mem. 114, 223-230 (2014).
- Dela Cruz, J. A., et al. Roles of dopamine D1 and D2 receptors in the acquisition and expression of fat-conditioned flavor preferences in rats. Neurobiol. Learn. Mem. 97, 332-337 (2012).
- Yu, W. Z., Silva, R. M., Sclafani, A., Delamater, A. R., Bodnar, R. J. Pharmacology of flavor preference conditioning in sham-feeding rats: effects of dopamine receptor antagonists. Pharmacol. Biochem. Behav. 65, 635-647 (2000).
- Yu, W. Z., Silva, R. M., Sclafani, A., Delamater, A. R., Bodnar, R. J. Role of D(1) and D(2) dopamine receptors in the acquisition and expression of flavor-preference conditioning in sham-feeding rats. Pharmacol. Biochem. Behav. 67 (1), 537-544 (2000).
- Touzani, K., Bodnar, R. J., Sclafani, A. Activation of dopamine D1-like receptors in nucleus accumbens is critical for the acquisition, but not the expression, of nutrient-conditioned flavor preferences in rats. Eur. J. Neurosci. 27, 1525-1533 (2008).
- Touzani, K., Bodnar, R. J., Sclafani, A. Dopamine D1-like receptor antagonism in amygdala impairs the acquisition of glucose-conditioned flavor preference in rats. Eur. J. Neurosci. 30, 289-298 (2009).
- Touzani, K., Bodnar, R. J., Sclafani, A. Acquisition of glucose-conditioned flavor preference requires the activation of dopamine D1-like receptors within the medial prefrontal cortex in rats. Neurobiol. Learn. Mem. 94, 214-219 (2010).
- Malkusz, D. C., et al. Dopamine signaling in the medial prefrontal cortex and amygdala is required for the acquisition of fructose-conditioned flavor preferences in rats. Behav. Brain Res. 233, 500-507 (2012).
- Bernal, S. Y., et al. Role of dopamine D1 and D2 receptors in the nucleus accumbens shell on the acquisition and expression of fructose-conditioned flavor-flavor preferences in rats. Behav. Brain Res. 190, 59-66 (2008).
- Bernal, S. Y., et al. Role of amygdala dopamine D1 and D2 receptors in the acquisition and expression of fructose-conditioned flavor preferences in rats. Behav. Brain Res. 205, 183-190 (2009).
- Dragunow, M., Faull, R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J. Neurosci. Methods. 29, 261-265 (1989).
- VanElzakker, M., Fevurly, R. D., Breindel, T., Spencer, R. L. Environmental novelty is associated with a selective increase in Fos expression in the output elements of the hippocampal formation and the perirhinal cortex. Learn. Mem. 15, 899-908 (2008).
- Norgren, R., Hajnal, A., Mungarndee, S. S. Gustatory reward and the nucleus accumbens. Physiol. Behav. 89, 531-535 (2006).
- Park, T. H., Carr, K. D. Neuroanatomical patterns of fos-like immunoreactivity induced by a palatable meal and meal-paired environment in saline- and naltrexone-treated rats. Brain Res. 805, 169-180 (1998).
- Zhao, X. L., Yan, J. Q., Chen, K., Yang, X. J., Li, J. R., Zhang, Y. Glutaminergic neurons expressing c-Fos in the brainstem and amygdala participate in signal transmission and integration of sweet taste. Nan.Fang Yi.Ke.Da.Xue.Xue.Bao. 31, 1138-1142 (2011).
- Mungarndee, S. S., Lundy, R. F., Norgren, R. Expression of Fos during sham sucrose intake in rats with central gustatory lesions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R751-R763 (2008).
- Otsubo, H., Kondoh, T., Shibata, M., Torii, K., Ueta, Y. Induction of Fos expression in the rat forebrain after intragastric administration of monosodium L-glutamate, glucose and NaCl. Neurosci. 196, 97-103 (2011).
- Yamamoto, T., Sako, N., Sakai, N., Iwafune, A. Gustatory and visceral inputs to the amygdala of the rat: conditioned taste aversion and induction of c-fos-like immunoreactivity. Neurosci. Lett. 226, 127-130 (1997).
- Mitra, A., Lenglos, C., Martin, J., Mbende, N., Gagne, A., Timofeeva, E. Sucrose modifies c-fos mRNA expression in the brain of rats maintained on feeding schedules. Neurosci. 192, 459-474 (2011).
- Pecoraro, N., Dallman, M. F. c-Fos after incentive shifts: expectancy, incredulity, and recovery. Behav. Neurosci. 119, 366-387 (2005).
- Hamlin, A. S., Blatchford, K. E., McNally, G. P. Renewal of an extinguished instrumental response: Neural correlates and the role of D1 dopamine receptors. Neurosci. 143, 25-38 (2006).
- Kerfoot, E. C., Agarwal, I., Lee, H. J., Holland, P. C. Control of appetitive and aversive taste-reactivity responses by an auditory conditioned stimulus in a devaluation task: A FOS and behavioral analysis. Learn. Mem. 14, 581-589 (2007).
- Zhang, M., Kelley, A. E. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neurosci. 99, 267-277 (2000).
- Teegarden, S. L., Scott, A. N., Bale, T. L. Early life exposure to a high fat diet promotes long-term changes in dietary preferences and central reward signaling. Neurosci. 162, 924-932 (2009).
- Del Rio, D., et al. Involvement of the dorsomedial prefrontal cortex in high-fat food conditioning in adolescent mice. Behav. Brain Res. 283, 227-232 (2015).
- Knapska, E., Radwanska, K., Werka, T., Kaczmarek, L. Functional internal complexity of amygdala: focus on gene activity mapping after behavioral training and drugs of abuse. Physiol. Rev. 87, 1113-1173 (2007).
- Dela Cruz, J. A. D., et al. c-Fos induction in mesotelencephalic dopamine pathway projection targets and dorsal striatum following oral intake of sugars and fats in rats. Brain Res. Bull. 111, 9-19 (2015).
- Paxinos, G., Watson, C. . The rat brain in stereotaxic coordinates. , (2006).
- Ranaldi, R., et al. The effects of VTA NMDA receptor antagonism on reward-related learning and associated c-fos expression in forebrain. Behav. Brain Res. 216, 424-432 (2011).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone