Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Protocol for Isolating the Mouse Circle of Willis
W tym Artykule
Podsumowanie
We describe here a reproducible protocol for isolating the mouse circle of Willis.
Streszczenie
The cerebral arterial circle (circulus arteriosus cerebri) or circle of Willis (CoW) is a circulatory anastomosis surrounding the optic chiasma and hypothalamus that supplies blood to the brain and surrounding structures. It has been implicated in several cerebrovascular disorders, including cerebral amyloid angiopathy (CAA)-associated vasculopathies, intracranial atherosclerosis and intracranial aneurysms. Studies of the molecular mechanisms underlying these diseases for the identification of novel drug targets for their prevention require animal models. Some of these models may be transgenic, whereas others will involve isolation of the cerebro-vasculature, including the CoW.The method described here is suitable for CoW isolation in any mouse lineage and has considerable potential for screening (expression of genes, protein production, posttranslational protein modifications, secretome analysis, etc.) studies on the large vessels of the mouse cerebro-vasculature. It can also be used for ex vivo studies, by adapting the organ bath system developed for isolated mouse olfactory arteries.
Wprowadzenie
The cerebral arterial circle (circulus arteriosus cerebri), also known as the circle of Willis (CoW), loop of Willisor Willis polygon) was first described by Thomas Willis in 1664. It is a circulatory anastomosis located around the optic chiasma and hypothalamus that can be considered as a central hub supplying blood to the brain and surrounding structures. Blood enters this structure via the internal carotid and vertebral arteries and it flows out of the circle via the interior middle and posterior cerebral arteries. Each of these arteries has left and right branches on either side of the circle. The basilar, post communicating, and anterior communicating arteries complete the circle (Figure 1 and Figure 2). The risk of impaired blood flow in any of the outflow arteries is minimized by the merging of blood entering the circle from the carotid and cerebral arteries, thereby guaranteeing that sufficient blood is supplied to the brain. This structure also serves as the main route for collateral blood flow in severe occlusive diseases of the internal carotid artery.
Several types of cerebrovascular disorders have their origin in the CoW. The most common are cerebral amyloid angiopathy (CAA)-associated vasculopathies, intracranial atherosclerosis and intracranial aneurysms. 1,2,3 These disorders may lead to hypoperfusion due to vasodilation, and intracerebral and/or subarachnoid hemorrhages ultimately translating into ischemic or hemorrhagic strokes or, at best, a transient ischemic attack. Recent advances in diagnostic procedures, including neuroimaging, possibly combined with angiography, have made it possible to diagnose these major cerebrovascular diseases clinically, without the need for a brain biopsy. Nevertheless, effective and specific treatments (pharmacological or endovascular) are currently lacking and there is therefore a need to define new molecular targets.
The identification of novel drug targets for the prevention of these diseases in humans will require animal models and ways of isolating the cerebro-vasculature including the CoW. Such models should provide evidence of and clues to the specific changes, including inflammatory changes, occurring in the walls of the large vessels in animal models of intracranial artery aneurysm, CAA or intracranial atherosclerosis. 4,5,6
We have established a method for mouse CoW isolation to facilitate studies of vessel inflammation in Alzheimer's disease (AD) and related diseases, such as CAA. This method for isolating the mouse CoW was developed for the assessment of inflammatory cerebrovascular gene expression during disease progression. Together with the detection of amyloid beta deposition within the walls of the leptomeningeal and pial arteries, this method could make it easier to determine the possible relationship between inflammatory gene expression in the cerebro-vasculature wall and Aβ-peptide accumulation. The vascular network of the brain, including the leptomeningeal and pial in the subarachnoid space, is an extension of the large arteries forming the circle of Willis. The method described here could be used to isolate the CoW of any mouse lineage and could be used for all types of screening (e.g., gene expression, protein production and posttranslational protein modifications) on the large vessels of the mouse cerebro-vasculature.
Protokół
All procedures were performed in accordance with European Community standards for the care and use of laboratory animals, with the approval of the local ethics committee for animal experimentation (Ile de France-Paris-Committee, Authorization 4270).
1. Anesthesia
- Infuse a lethal dose of pentobarbital (up to 1 mg/10 g body weight) intraperitoneally (27-gauge needle and 1-ml syringe) into adult mice before surgery.
2. Vessel Perfusion
NOTE: There is no need to apply vet ointment to the eyes during vessel perfusion. This procedure is rapid (5-10 minutes) and ends in the death of the animal. Confirm the lack of response with a toe pinch.
- Using iris scissors, make an incision, about 4 cm long, into the abdominal wall and peritoneum, just beneath the rib cage.
- Make a small incision (a few millimeters long) in the diaphragm and then continue the incision of the diaphragm along the entire length of the rib cage to expose the pleural cavity.
- Lift the sternum away and clamp the tip of the sternum with the hemostat; place the hemostat on the neck. Carefully trim the adipose tissue connecting the sternum to the heart.
- Pass the 15-gauge perfusion needle through the left ventricle into the apex of the heart.
- Finally, use scissors to cut one of the liver lobes to create an outlet.
NOTE: An alternative outlet can be created by using iris scissors to create an incision to the right atrium. - Perfuse the animal with 25 to 50 ml of phosphate-buffered saline (PBS) with a pump operating at a rate of 2.5 ml/min. The liver should blanch as the blood is replaced with PBS.
- After approximately five minutes, once the fluid from the liver is completely clear, stop the perfusion.
- If immunostaining or regular staining is planned, perfuse the animal with 50 ml of paraformaldehyde (PFA; 4% in PBS) for 15 min.
NOTE: Caution, PFA fumes are toxic. Perfusion of the animal with PFA should be carried out in a ventilated fume hood.
3. Isolation of the Brain and the Circle of Willis
- Isolation of the brain
- Remove the head with a pair of surgical scissors.
- Make a midline incision with iris scissors, along the skin from the neck to the nose.
- Trim off the skin to expose the skull and remove any residual muscles and adipose tissues with iris scissors.
- Place the sharp end of the iris scissors into the foramen magnum on one side and carefully slide them along the inner surface of the skull to the external auditory meatus (also known as the ear canal).
- Reproduce the incision described in 3.1.4 on the contralateral side and make a midline cut along the inner surface of the inter-parietal bone to the start of the sagittal suture.
- Plant the iris scissors in the frontal bone, right between the eyes, in the sagittal suture and then open them to split the skull in two.
- Lift out the brain, grabbing the olfactory bulbs and using the iris scissors to cut off the nerve connections on its ventral surface.
- Remove the brain and place it in a 60-mm Petri dish containing ice-cold PBS for CoW isolation. Completely immerse the brain in the PBS. If the brain was fixed with 4% PFA (for subsequent sectioning and immunostaining or regular staining), keep it in a bath of 4% PFA at 4 °C for 24 hr.
- Isolation of the circle of Willis
NOTE: A dissecting microscope is required for CoW isolation. The brain should be kept at 4 °C throughout the entire procedure.- Put the brain upside down (i.e., on its dorsal surface) to visualize the CoW.
- Use a small forceps to grab the anterior cerebral arteries (ACA) at the base of the olfactory lobes (a, Figure 1) and exert pressure to dissociate them from the vessel continuum. Use the same procedure to cut the middle cerebral arteries (MCA) in b (Figure 1).
- Use the sharp ends of the forceps to lift and remove the major arteries forming the CoW from the cortex.
- Lift up the start of the posterior communicating arteries (PCA) to disconnect them from the brain, by gripping the middle cerebral arteries (MCA) with the forceps. Pick up the anterior arteries (ACA and MCA) and pull them gently over the optic chiasm in an anterior-dorsal direction. To prevent disruption of the CoW, interrupt the the procedure to deal with the other arteries.
- Repeat steps 3.2.2 and 3.2.3 for the superior and posterior cerebellar arteries (SCA)/(PCA) (c, Figure 1) and for the basilar artery (BA) (d, Figure 1), pulling them in a dorsal-anterior direction. Stop at the end of the procedure described in 3.2.4.
- Remove the entire CoW by pulling gently with the forceps. Place the CoW in a 60-mm Petri dish filled with ice-cold PBS and remove any remaining attached brain tissue with two forceps, holding the CoW in place with small pins.
- Keep the harvested CoW at -80 °C for subsequent processing for RNA purification (RNA extraction yields large amounts of RNA — approximately 500 ng) or protein extraction.
Note: The CoW can be maintained ex vivo for 24 hr, by adapting the organ bath system developed for isolated mouse olfactory artery. 7

Figure 1: Schematic Diagram of a Ventral View of the Mouse Brain Highlighting the CoW. The CoW is formed from the two internal carotid arteries (MCA), which are derived from the two anterior cerebral arteries (ACA); the basilar artery (BA) branches into the posterior (PCA) and superior (SCA) cerebral arteries, and two vertebral arteries (VA).
Wyniki
The PBS-perfused mouse is killed and the CoW is isolated as described in section 3.2 of the protocol. When the dissection is performed correctly, the CoW should come out in one piece and should be slightly transparent due to the absence of residual blood in the vasculature.
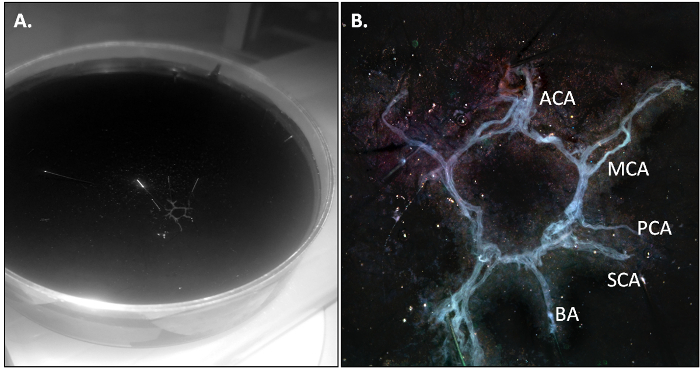
Figure 2: The Mouse CoW after Isolation. (A) Overv...
Dyskusje
We describe here a reproducible protocol for the isolation of the circle of Willis. The most common cerebrovascular disorders involving the CoW are CAA-associated vasculopathies, intracranial atherosclerosis and intracranial aneurysm, all of which affect the walls of arterial vessels. The risk factors are well known, but the molecular pathogenesis of these cerebral disorders remains poorly understood and specific biological markers for predicting their occurrence are lacking. There is considerable interest in methods for...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This work was supported by Paris VI University and a Pierre Fabre Innovation grant.
Materiały
| Name | Company | Catalog Number | Comments |
| Dulbecco’s Phosphate Buffered Saline | Sigma-Aldrich | D8537 | |
| Dumont #55 Forceps | Fine Science Tools | 11295-51 | |
| Hardened Fine Iris Scissors | Fine Science Tools | 14090-11 | |
| Scissors - Straight / Sharp / Sharp 16.5 cm | Fine Science Tools | 14002-16 | |
| Dumont #7b Forceps | Fine Science Tools | 11270-20 | |
| Stereoscopic Zoom Microscope | Nikon | SMZ745T | |
| CellBIND Surface 60mm Culture Dish | Corning | #3295 | |
| Peristaltic Pump - MINIPULS 3 | Gilson | M312 | |
| Pentobarbital Sodique | Ceva Santé Animale | FR/V/2770465 3/1992 |
Odniesienia
- Beckmann, N., et al. Age-dependent cerebrovascular abnormalities and blood flow disturbances in APP23 mice modeling Alzheimer's disease. J Neurosci. 23 (24), 8453-8459 (2003).
- Sadasivan, C., Fiorella, D. J., Woo, H. H., Lieber, B. B. Physical factors effecting cerebral aneurysm pathophysiology. Ann Biomed Eng. 41 (7), 1347-1365 (2013).
- Ritz, K., Denswil, N., Stam, O., van Lieshout, J., Daemen, M. Cause and mechanisms of intracranial atherosclerosis. Circulation. 130 (16), 1407-1414 (2014).
- Tulamo, R., Frösen, J., Hernesniemi, J., Niemelä, M. Inflammatory changes in the aneurysm wall: a review. J Neurointerv Surg. 2 (2), 120-130 (2009).
- Yamada, M. Cerebral amyloid angiopathy: emerging concepts. J Stroke. 17 (1), 17-30 (2015).
- Oy, B. Intracranial atherosclerotic stroke: specific focus on the metabolic syndrome and inflammation. Curr Atheroscler Rep. 8 (4), 330-336 (2006).
- Lee, H. J., Dietrich, H. H., Han, B. H., Zipfel, G. J. Development of an ex vivo model for the study of cerebrovascular function utilizing isolated mouse olfactory artery. J Korean Neurosurg Soc. 57 (1), 1-5 (2015).
- Hosaka, K., Downes, D. P., Nowicki, K. W., Hoh, B. L. Modified murine intracranial aneurysm model: aneurysm formation and rupture by elastase and hypertension. J Neurointerv Surg. 6 (6), 474-479 (2013).
- Gauthier, S. A., Sahoo, S., Jung, S. S., Levy, E. Murine cerebrovascular cells as a cell culture model for cerebral amyloid angiopathy: isolation of smooth muscle and endothelial cells from mouse brain. Methods Mol Biol. 849, 261-274 (2012).
- Choi, S., Kim, J., Kim, K., Suh, S. Isolation and in vitro culture of vascular endothelial cells from mice. Korean J Physiol Pharmacol. 19 (1), 35-42 (2015).
- Peters, D. G., Kassam, A. B., Yonas, H., O'Hare, E. H., Ferrell, R. E., Brufsky, A. M. Comprehensive transcript analysis in small quantitiesof mRNA by SAGE-Lite. Nucleic Acids Res. 27 (24), (1999).
- Badhwar, A. Stanimirovic, Hamel, & Haqqani The proteome of mouse cerebral arteries. J Cereb Blood Flow Metab. 34 (6), 1033-1046 (2014).
- Castro, L., Brito, M., et al. Striatal neurones have a specific ability to respond to phasic dopamine release. J Physiol. 591 (13), 3197-3214 (2013).
- Hübscher, D., Nikolaev, V. Generation of transgenic mice expressing FRET biosensors. Methods Mol Biol. 1294, 117-129 (2015).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone