Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Ice Generation and the Heat and Mass Transfer Phenomena of Introducing Water to a Cold Bath of Brine
W tym Artykule
Podsumowanie
Here, we present a protocol to demonstrate the generation of ice when water is introduced to a cold bath of brine, as a secondary refrigerant, at a range of temperatures well below the freezing point of water. It can be used as an alternative way of producing ice for industry.
Streszczenie
We demonstrate a method for the study of the heat and mass transfer and of the freezing phenomena in a subcooled brine environment. Our experiment showed that, under the proper conditions, ice can be produced when water is introduced to a bath of cold brine. To make ice form, in addition to having the brine and water mix, the rate of heat transfer must bypass that of mass transfer. When water is introduced in the form of tiny droplets to the brine surface, the mode of heat and mass transfer is by diffusion. The buoyancy stops water from mixing with the brine underneath, but as the ice grows thicker, it slows down the rate of heat transfer, making ice more difficult to grow as a result. When water is introduced inside the brine in the form of a flow, a number of factors are found to influence how much ice can form. Brine temperature and concentration, which are the driving forces of heat and mass transfer, respectively, can affect the water-to-ice conversion ratio; lower bath temperatures and brine concentrations encourage more ice to form. The flow rheology, which can directly affect both the heat and mass transfer coefficients, is also a key factor. In addition, the flow rheology changes the area of contact of the flow with the bulk fluid.
Wprowadzenie
Ice slurries are extensively used in industry, and one particularly successful application is the ice-pigging technology1,2. In comparison to the conventional foam and solid pig, the ice pig can travel through complex topologies over a long distance because of the lubrication effect of the liquid phase and the elevation of its freezing point as some of the ice crystals melt3,4,5. Even if the pig gets stuck, one can simply wait for the ice slurries to melt and resume the cleaning process later. This method of pipe cleaning is cheap and easy to use.
The ice fraction plays a key role in the performance of the ice pig. To measure the ice fraction, one can use a cafetière (French press) to determine if the ice slurry is thick enough6,7. A high cafetière ice fraction, typically 80%, is required when carrying out ice pigging. Recent research on online ice fraction detection showed that both electromagnetic and ultrasonic waves are suitable for the job8,9,10,11.
The ice pig is usually made by a scraped-surface ice maker from a 5 wt% NaCl solution (brine). It is also the primary way of making ice slurries in industry. This type of ice maker freezes water or brine onto a cold metallic surface, typically a smooth 316 steel surface and then cyclically shears the ice particles off. The liquid-to-metal interfaces are very complex and are affected by a broad range of factors that are essential to ice making12. The interface between non-metal and water can be very different, and one especially interesting example is Kaolinite. The Kaolinite-water interface is special because there is not a favorable ice structure adjacent to the solid's surface, but rather a layer of amphoteric substrate fluid that encourages the ice-like hydrogen-bonded clusters to form on top of it13,14. Another way of producing the ice pig requires crushing the premade ice blocks while high-concentration brine is added simultaneously. For this method, the refrigeration system can run at a much higher evaporating temperature because no freezing point depressant (FPD) is added prior to the formation of ice; it is hence considered more efficient due to the lowered compression ratio and lessened power for a given cooling duty15,16,17.
There are two other ice production methods: producing ice from supercooled water and putting refrigerant and water in direct contact18,19. The supercooling method involves disturbing the metastable supercooled water to generate ice nucleation and growth. The biggest problem for this method is the unwanted ice formation that can block the system. The direct contact method is considered not suitable for ice pigging because neither refrigerant nor lubrication oil are wanted in the final ice product.
The formation of ice requires heat and mass transfer due to the latent heat of fusion generated in the process. It was first discovered by Osborn Reynolds in 1874 that the transportation of heat and mass in gases are strongly coupled and can be expressed in similar mathematical formulae20. This work formed the pioneering paper on the subject of momentum, heat, and mass transfer in fluids and was reprinted several times21,22. This subject was then studied by a number of others, from both analytical and empirical approaches, for gases, liquids, and molten metal23,24,25,26,27,28,29,30,31,32,33. Aside from the heat and mass transfer, the fluid needs nucleation sites where dendritic ice growth can develop. A modern insight into the growth of ice crystals uses Constructal Law, developed by Adrian Bejan, to explain why ice grows in this way34,35,36.
The ice formation in brine is very different from that in pure water due to the existence of salt. First of all, salt changes the thermodynamics of the fluid and depresses its freezing point. Secondly, salt cannot dissolve in the ice matrix (except for hydrohalite, which can only form when the temperature reaches the eutectic point), and it is rejected to the bulk fluid when ice starts to grow. The rejection of salt was discovered in both sea ice and ice studied in the lab37,38. Since the rejected high-concentration brine is at a temperature well below the freezing point of sea water, as it descends, ice grows at the interface between the flowing brine and the quiescent bulk fluid. These ice stalactites, also named brinicles, were first discovered in McMurdo Sound, Antarctica and were studied experimentally39,40,41,42. In 2011, BBC filmed the formation of brinicles in its Frozen Planet series43,44.
In our lab, it was discovered that by reversing the flowing and quiescent fluids when water is introduced to a bath of cold brine, the water may transform into ice under the correct conditions45. It was found that the location where the water is introduced, flow rheology, and brine temperature and concentration are all key factors influencing how much ice can be produced. The overall goal of this study is to investigate if an ice maker can be developed through this mechanism to generate ice slurries, considering that the elevated evaporator temperature and the high rate of liquid-to-liquid heat transfer can enhance the efficiency of energy usage. This article shares key aspects of the experiment.
Protokół
Caution: There are two poisonous chemicals, methanol and ethylene glycol, used in these experiments. Methanol can be metabolized in the human body to generate formaldehyde and then to formic acid or formate salt. These substances are poisonous to the central nervous system and may even cause death. Ethylene glycol can be oxidized to glycolic acid, which can then turn into oxalic acid. This can cause kidney failure and death. Do not drink these chemicals. Consult a doctor immediately if an accident occurs.
1. The Cooling System
NOTE: It is very difficult to keep the brine at -18 °C or so when the ambient temperature is roughly at room temperature. It is important that the tanks storing the ethylene glycol and brine are well-insulated and of a reasonable size to avoid excess electricity consumption and to ensure optimal system performance. It is recommended that the tank size does not exceed 30 L.
- Prepare the secondary cooling fluid
- Pour 1 L of ethylene glycol into the secondary cooling tank, Tank A (Base: 400 mm × 200 mm, Height: 350 mm). Add roughly 0.6-0.65 L (600-650 g) of water to Tank A.
- Repeat step 1.1.1 several times until there is enough fluid in Tank A (25 L).
- Stir the fluid so that the fluid is homogeneous.
- Switch on the two pumps in Tank A to the full-capacity setting (2,500 L/h). Make sure that all bubbles trapped in the heat exchangers and pipes are released.
- Switch off the pump to observe if all the bubbles are released. If not, repeat step 1.1.4.
- Preparation of the brine
NOTE: In this example, 22 wt% brine is prepared. If other concentrations are needed, the mass of salt added should also be changed accordingly. The reference brine concentration and density values can be found on Page D-257 of the 64th Edition (1983) of the CRC Handbook of Chemistry and Physics46.- Add 4 kg of water into a 5-L plastic beaker.
- Measure 1 kg of NaCl salt on an electronic scale and pour this salt into the beaker with the water.
- Stir the mixture until the solution is clear (i.e., there are no salt particles or water bubbles visible in the solution).
- Take a sample, ~10 mL, of the solution using a 10-mL syringe.
- Inject the fluid into the U-tube density meter.
- Check for air bubbles in the tube. If there are any, inject more fluid to push them out.
- Press "Quick Settings" and select "Density Temperature." Type in 20 °C and press "OK." The density meter will now measure the fluid density at this temperature.
- Press start and wait for the result.
- Compare the density reading with 1164.00 kg/m3.
- Add more salt if the reading is below the comparison density. Add water if otherwise.
- Repeat steps 1.2.3-1.2.10 until the fluid density is correct (1164.00 kg/m3).
- Pour this solution into a larger container, Container A.
- Use steps 1.2.1-1.2.12 to make 35-40 L of brine and put Container A into a chest freezer at -40 °C. Keep the brine there for 48-72 h, until its temperature reaches -19.18 °C (freezing point of this 22 wt% brine).
2. Preparation of the Ice for the Injecting and Washing Water
- Prepare ice for the injecting water
- Pour 1 L of water into a small container (200 × 200 × 50 mm).
- Repeat step 2.1.1 with another container and place the two containers in the chest freezer at -40 °C.
- Keep them in the freezer for 10 h or more to ensure that all of the water is frozen.
- Prepare the washing water's ice shell
- Fill a 5-L beaker with 5 L of water.
- Fill a 2-L beaker with 2 L of water.
- Place both beakers into the chest freezer at -40 °C for 8-10 h so that there is a thick shell of ice wrapping around unfrozen water.
- Use a high-velocity water jet at a speed of 3-5 m/s from the tap to open up a 3-cm diameter hole at the top of the ice shell.
- Drain the water inside the ice shell.
- Put the two beakers back in the freezer.
- If the mass of the ice shell does not reach 3 kg and 1 kg for the two beakers, respectively, repeat steps 2.2.1-2.2.5, but keep the beakers in the freezer longer in step 2.2.3. The two beakers should now be able to contain 2 L and 1 L of water, respectively.
3. Water Introduction Position and the Rheology Control Experiment
- Introduce water at the brine surface
- Decant 2 L of 22 wt% cold brine from Container A into the aluminum bucket of the ice cream maker and switch on the cooling unit.
- Measure the temperature of the brine with a thermometer/thermocouple (either K-Type or T-type are suitable). Carry on the experiment if the brine is -15 °C or lower.
- Fill up the 100-mL glass syringe with tap water at room temperature. Attach a 2-mm internal diameter, 1-mm thick, and 1-m long silicone tube to the tip of the syringe.
- Place the syringe at a specific position such that there is a head between the water in the syringe and the exit of the silicone tube. The hydrostatic pressure will squeeze the water out of the tube.
- Submerge a certain length of the silicone tube, typically 70 cm, into the brine.
- Adjust the relative position between the syringe and the tube exit so that the hydrostatic pressure is great enough to allow water to leave the syringe. If the tube is blocked, increase the head by elevating the syringe to a higher vertical position, until the hydrostatic pressure can overcome the shear stress within the tube.
- Keep the tube exit roughly 1 cm or less above the brine surface.
- Adjust the length of submerged tube and the syringe height to control the water's outlet let temperature and flow rate in order to determine how much ice can be made or how much mixing occurs at the brine surface. The freezing phenomenon should now be observed at the brine surface. See Reference 45 for further direction.
- Introduce water through the brine
- Repeat steps 3.1.1-3.1.6.
- Keep the tube exit inside the brine, preferably at the bottom of the container.
- Adjust the length of the submerged tube and the syringe height.
- Adjust the angle of the tube exit to control the rheology of the flow.
- Repeat steps 3.2.3-3.2.4 to find the best coupled flow rheology and flow rate that can produce the most ice.
4. Ice Production, Collection, and Measurement
- Make ice
- If there are bubbles in the pipes, switch on the two pumps inside Tank A to release the bubbles out of the glycol circulation system, and then switch off the pumps.
- Switch on the three refrigeration units and let them run for 10-16 h to cool down the ethylene glycol solutions.
- Measure the ethylene glycol solution with a thermometer/thermocouple. The glycol temperature should be at about -25 °C.
- Measure the temperature of the brine in Container A to make sure it is at -19 °C before proceeding to step 4.1.5.
- Fill the brine tank, Tank B, with roughly 30 L of brine from Container A and switch on the two pumps in Tank A.
- Measure the temperature of the glycol in Tank A. If it is colder than -19 °C, switch off one or more cooling units to prevent the precipitation of ice particles outside the heat exchangers in Tank B. If the temperature is warmer than the expected brine temperature, turn on all three cooling units. Carry out the experiment at -17 °C to -19 °C.
- Place the two premade blocks of ice from step 2.1 into the insulated 5-L beaker, Container B, and pour roughly 3 L of water into the beaker.
- Measure the water temperature and keep it at 2 °C by stirring the mixture between experiments if the temperature rises.
- Fill the glass syringe with 100 mL of the 2 °C water.
- Apply 5-10 mL of methanol to the glass window of Tank B to stop condensation and the formation of ice.
- Inject the water into the brine by adjusting the relative position between the syringe and the tube's exit so that there is a constant hydrostatic pressure and thus a constant flow rate. About 70 cm of the silicone tube should be submerged in the brine. Adjust the angle of injection to 0° so that the initial water velocity in the upward direction is 0 m/s.
NOTE: The syringe can be either hand-held or clamped to a stand. Hand-held is more appropriate when the brine temperature is colder, because it takes more time to adjust a stand, and ice may block the tube. Keep the flow rheology consistent throughout the experiment by ensuring a constant flow rate and injection angle (0°) and by keeping the freezing frontier roughly 3 cm above the tube exit. Do not let the flow enter the region where it starts to turn turbulent47. See Reference 45 for further direction. - Collect the ice as described in steps 4.2 and 4.3. Repeat steps 4.1.8 - 4.1.11 at different brine temperatures.
- Collect the produced ice and estimate how much ice is produced (dry collection)
- Put a container (200 × 200 × 50 mm) on the scale and zero the reading by pressing the "Turn On" button.
- Use the sieve to scoop out the ice and shake off the brine.
- Put this ice in the container. Measure the mass of the ice using the scale.
- After the ice is melted, use the 10-mL syringe to take a sample. Inject this sample of liquid into the density meter.
- Perform steps 1.2.6-1.2.9.
- Record the density reading.
- Calculate the net water mass from its density (i.e., the mass of water converted into ice) using the following formula:
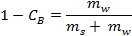
where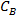 is the brine concentration by wt% and
is the brine concentration by wt% and 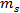 and
and 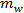 are the masses of salt and water, respectively.
are the masses of salt and water, respectively.
- Collect the produced ice and estimate how much ice is produced (wet collection)
- Fill the 5-L beaker with an ice shell (step 2.2) and room-temperature tap water. Put it back into the freezer at -40 °C.
- Decant the water with the ice shell from the 5-L beaker into a 2-L beaker when its temperature is at 0 °C. Fill up the 5-L beaker. Keep both beakers in the freezer.
- Scoop out the ice produced in steps 4.1.8 and 4.1.9 and pour 200-500 mL of water from the 2-L beaker onto the ice to wash it. Do not shake the sieve before applying the 0 °C water.
- Shake off the fluid in the sieve.
- Repeat steps 4.2.2-4.2.7.
Wyniki
Figure 1 compares the effects of water introduced at the brine surface to water injected through the brine. In the "ice-cap" scenario, the formed ice is solid because the water did not mix much with the bulk fluid. The temperature and density difference between the two fluids generates buoyancy force on the water and prevents them from mixing. Both fluids are static (i.e., the heat transfer is much greater than that of the mass; Sc ≈ 500, Pr ≈...
Dyskusje
The process of ice generation using brine as a secondary refrigerant involves the combination of heat and mass transfer. If the heat transfer is greater, then ice forms before the water has the chance to mix with the bulk fluid. It was observed that when there is a relative movement between the introduced water and the quiescent bulk brine (i.e., injecting water within the brine), the flow helps the heat transfer and encourages ice to form rapidly. However, when there is too much turbulence in the flow, no ice c...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The authors have no acknowledgements.
Materiały
| Name | Company | Catalog Number | Comments |
| DMA 4500 M | Anton Paar | 81546022 | Density Metre |
| GELATO Chef 2200 | magimix | 0036500504R13 | Ice Cream Maker |
| 280D | FREEZE MASTER | 241-1441 | Pipe Freezer |
| M17.5X2 | BLUE ICE MACHINES | GK924 | Slushy Puppy Machine |
| HH68K | OMEGA | 140045 | Thermometer |
| OHAUS | TS4KW | 1324 | Scale |
| ZFC321WA/BNI225 | ZANUSSI | 920672574-00 | Freezer |
| EIS Heater Matrix | Vauxhall | 214720041 | Heat Exchanger |
| 2500LPH | JBA | AP-2500 | Pump |
| Glass syringe | FORTUNA Optima | 100 mL | |
| OAT concentrated coolant | wilko | P30409014 | Ethylene Glycol |
| pure dried vacuum salt | INEOS Enterprise | 1433324 | NaCl Salt |
| Methylated Spirits | Barrettine | 1170 | Methanol |
Odniesienia
- Quarini, G. L. Cleaning and separation in conduits. UK patent. , (2001).
- Quarini, J. Ice-pigging to reduce and remove fouling and to achieve clean-in-place. Appl. Therm. Eng. 22, 747-753 (2002).
- Evans, T. S., Quarini, G. L., Shire, G. S. F. Investigation into the transportation and melting of thick ice slurries in pipes. Int. J. Refrig. 31, 145-151 (2008).
- Shire, G. S. F., Quarini, G. L., Rhys, T. D. L., Evans, T. S. The anomalous pressure drop behaviour of ice slurries flowing through constrictions. Int. J. Multiph. Flow. 34, 510-515 (2008).
- Shire, G. S. F., Quarini, G. L., Evans, T. S. Pressure drop of flowing ice slurries in industrial heat exchangers. Appl. Therm. Eng. 29, 1500-1506 (2009).
- Evans, T. S. . Technical Aspects of Pipeline Pigging with Flowing Ice Slurries [dissertation]. , (2007).
- Shire, G. S. F. . The behaviour of ice pigging slurries [dissertation]. , (2006).
- Hales, A., et al. Ice fraction measurement of ice slurries through electromagnetic attenuation. Int. J. Refrig. 47, 98-104 (2014).
- Hales, A., et al. The effect of salinity and temperature on electromagnetic wave attenuation in brine. Int. J. Refrig. 51, 161-168 (2015).
- Hales, A. . Ice slurry diagnostics through electromagnetic wave attenuation and other techniques [dissertation]. , (2015).
- Lucas, E. J. K., Hales, A., McBryde, D., Yun, X., Quarini, G. L. Noninvasive Ultrasonic Monitoring of Ice Pigging in Pipes Containing Liquid Food Materials. J. Food Process. Eng. 40, e12306 (2015).
- Carrasco, J., Hodgson, A., Michaelides, A. A molecular perspective of water at metal interfaces. Nat. Mater. 11, 667-674 (2012).
- Hu, X. L., Michaelides, A. Ice formation on kaolinite: Lattice match or amphoterism? . Surf. Sci. 601, 5378-5381 (2007).
- Hu, X. L., Michaelides, A. The kaolinite (0 0 1) polar basal plane. Surf. Sci. 604, 111-117 (2010).
- Leiper, A. N., Ash, D. G., McBryde, D. J., Quarini, G. L. Improving the thermal efficiency of ice slurry production through comminution. Int. J. Refrig. 35, 1931-1939 (2012).
- Leiper, A. . Carnot cycle optimisation of ice slurry production through comminution of bulk ice [dissertation]. , (2012).
- Leiper, A. N., Hammond, E. C., Ash, D. G., McBryde, D. J., Quarini, G. L. Energy conservation in ice slurry applications. Appl. Therm. Eng. 51, 1255-1262 (2013).
- Bédécarrats, J. -. P., David, T., Castaing-Lasvignottes, J. Ice slurry production using supercooling phenomenon. Int. J. Refrig. 33, 196-204 (2010).
- Wijeysundera, N. E., Hawlader, M. N. A., Andy, C. W. B., Hossain, M. K. Ice-slurry production using direct contact heat transfer. Int. J. Refrig. 27, 511-519 (2004).
- Reynolds, O. On the extent and action of the heating surface of steam boilers. Proc. Lit. Philos. Soc. Manch. 14, 7-12 (1874).
- Reynolds, O. . Papers on mechanical and physical subjects: reprinted from various transactions and journals. , 81-85 (1900).
- Reynolds, O. Papers on mechanical and physical subjects. Int. J. Heat Mass Transfer. 12, 129-136 (1969).
- Prandtl, L. Eine Beziehung zwischen Wärmeaustausch und Strömungswiderstand der Flüssigkeiten (On the relation between heat exchange and stream resistance of fluid flow). Physik. Z. 11, 1072-1078 (1910).
- Prandtl, L. Bemerkung über den Wärmeübergang im Rohr (Note on heat transmission in pipes). Physik. Z. 29, 487-489 (1928).
- Taylor, G. I. Conditions at the surface of a hot body exposed to the wind. Rep. Memo. ACA. 272, (1916).
- Taylor, G. I. The Application of Osborne Reynolds' Theory of Heat Transfer to Flow through a Pipe. Proc. R. Soc. A. 129, 25-30 (1930).
- Kármán, T. v. . Proceedings of the Fourth International Congress for Applied Mechanics. , 54-91 (1934).
- Kármán, T. v. The analogy between fluid friction and heat transfer. Trans. Am. Soc. Mech. Eng. 61, 705-710 (1939).
- Martinelli, R. C. Heat transfer to molten metals. Trans. Am. Soc. Mech. Eng. 69, 947-959 (1947).
- Colburn, A. P. A method of correlating forced convection heat-transfer data and a comparison with fluid friction. Trans. Am. Inst. Chem. Eng. 29, 174-210 (1933).
- Colburn, A. P. A method of correlating forced convection heat-transfer data and a comparison with fluid friction. Int. J. Heat Mass Transfer. 7, 1359-1384 (1964).
- Chilton, T. H., Colburn, A. P. Mass Transfer (Absorption) Coefficients Prediction from Data on Heat Transfer and Fluid Friction. Ind. Eng. Chem. 26, 1183-1187 (1934).
- Friend, W. L., Metzner, A. B. Turbulent heat transfer inside tubes and the analogy among heat, mass, and momentum transfer. AIChE J. 4, 393-402 (1958).
- Bejan, A. Constructal-theory network of conducting paths for cooling a heat generating volume. Int. J. Heat Mass Transfer. 40, 799-816 (1997).
- Bejan, A., Lorente, S. Constructal theory of generation of configuration in nature and engineering. J. Appl. Phys. 100, 041301 (2006).
- Bejan, A., Lorente, S., Yilbas, B. S., Sahin, A. Z. Why solidification has an S-shaped history. Sci. Rep. 3, 1711 (2013).
- Lake, R. A., Lewis, E. L. Salt rejection by sea ice during growth. J. Geophys. Res. 75, 583-597 (1970).
- Wettlaufer, J. S., Worster, M. G., Huppert, H. E. Natural convection during solidification of an alloy from above with application to the evolution of sea ice. J. Fluid Mech. 344, 291-316 (1997).
- Paige, R. A. Stalactite Growth beneath Sea Ice. Science. 167, 171-172 (1970).
- Dayton, P. K., Martin, S. Observations of ice stalactites in McMurdo Sound, Antarctica. J. Geophys. Res. 76, 1595-1599 (1971).
- Eide, L. I., Martin, S. The formation of brine drainage features in young sea ice. J. Glaciol. 14, 137-154 (1975).
- Martin, S. Ice stalactites: comparison of a laminar flow theory with experiment. J. Fluid Mech. 63, 51-79 (1974).
- Jeffs, K., Attenborough, D. . Frozen Planet: Episode 5 'Winter'. , (2011).
- Fothergill, A., Berlowitz, V., Attenborough, D. Ch. Winter: Life closes down. in Frozen Planet: A World Beyond Imagination. , (2011).
- Yun, X., et al. Ice formation in the subcooled brine environment. Int. J. Heat Mass Transfer. 95, 198-205 (2016).
- Weast, R. C. . CRC Handbook of Chemistry and Physics. 64, 257-258 (1983).
- Bejan, A., Lage, J. L. The Prandtl Number Effect on the Transition in Natural Convection Along a Vertical Surface. J. Heat Transfer. 112, 787-790 (1990).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone