Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Inducible T7 RNA Polymerase-mediated Multigene Expression System, pMGX
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
This study describes methods for the T7-mediated co-expression of multiple genes from a single plasmid in Escherichia coli using the pMGX plasmid system.
Streszczenie
Co-expression of multiple proteins is increasingly essential for synthetic biology, studying protein-protein complexes, and characterizing and harnessing biosynthetic pathways. In this manuscript, the use of a highly effective system for the construction of multigene synthetic operons under the control of an inducible T7 RNA polymerase is described. This system allows many genes to be expressed simultaneously from one plasmid. Here, a set of four related vectors, pMGX-A, pMGX-hisA, pMGX-K, and pMGX-hisK, with either the ampicillin or kanamycin resistance selectable marker (A and K) and either possessing or lacking an N-terminal hexahistidine tag (his) are disclosed. Detailed protocols for the construction of synthetic operons using this vector system are provided along with the corresponding data, showing that a pMGX-based system containing five genes can be readily constructed and used to produce all five encoded proteins in Escherichia coli. This system and protocol enables researchers to routinely express complex multi-component modules and pathways in E. coli.
Wprowadzenie
Co-expression of multiple proteins is increasingly essential, particularly in synthetic biology applications, where multiple functional modules must be expressed1; in studying protein-protein complexes, where expression and function often require co-expression2,3; and in characterizing and harnessing biosynthetic pathways, where each gene in the pathway must be expressed4,5,6,7,8. A number of systems have been developed for co-expression, particularly in the host organism Escherichia coli, the work horse for laboratory recombinant protein expression9. For example, multiple plasmids with differing selectable markers can be used to express individual proteins using a wealth of different expression vectors10,11. Single plasmid systems for multiple protein expression have used either multiple promoters to control the expression of each gene10,12; synthetic operons, where multiple genes are encoded on a single transcript2,13; or, in some cases, a single gene encoding a polypeptide that is ultimately proteolytically processed, yielding the desired proteins of interest14.
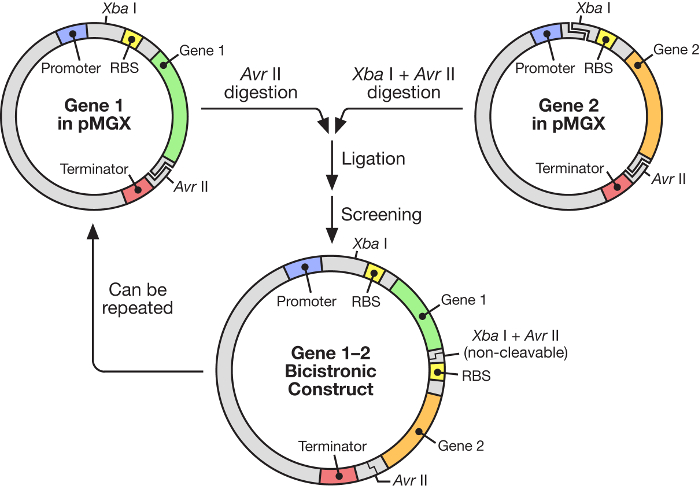
Figure 1: pMGX workflow showing the construction of a polycistronic vector. The pMGX system provides a flexible, easy-to-use strategy for the construction of synthetic operons under the control of an inducible T7 promoter. Please click here to view a larger version of this figure.
In this manuscript, the use of a highly effective system for the construction of multigene synthetic operons under the control of an inducible T7 RNA polymerase (Figure 1) is described. This system allows many genes to be expressed simultaneously from one plasmid. It is based on a plasmid system, originally called pKH22, that has been used successfully for a number of different applications6,7,8. Here, this plasmid set is expanded to include four related vectors: pMGX-A, an expression vector lacking any C- or N-terminal tags and with the ampicillin resistance marker; pMGX-hisA, an expression vector encoding an N-terminal hexahistidine tag and with the ampicillin resistance marker; pMGX-K, an expression vector lacking any C- or N-terminal tags and with the kanamycin resistance marker; and pMGX-hisK, an expression vector encoding an N-terminal hexahistidine tag and with the kanamycin resistance marker. In this study, the method for generating a polycistronic vector containing five genes using the pMGX system, specifically pMGX-A, is demonstrated along with the successful production of each individual protein in Escherichia coli.
Access restricted. Please log in or start a trial to view this content.
Protokół
1. Obtaining Genes of Interest
- Design synthetic genes.
- Optimize a gene sequence for E. coli expression.
- Remove any problematic restriction sites from the sequence (AvrII, NdeI, EcoRI, and XbaI).
- Incorporate restriction sites for cloning; a 5′-NdeI site and a 3′-EcoRI site are recommended. Other sites can be used, if necessary; refer to the multicloning region of the selected plasmid (Figure S1-S4). If desired, include a 5′ or 3′ encoding tag for Western blot detection.
- Commercially order the designed gene.
- Either blunt clone the gene into a blunt vector using a blunt cloning kit, as per the manufacturer's instructions; design primers to amplify the gene (then proceed to Step 1.2.2); or add additional 5′ and 3′ ends for direct cloning into a pMGX plasmid and proceed to Step 2. Here, the representative results are from blunt cloning the genes of interest.
- Amplify the desired gene (from a designed and optimized synthetic gene or from template DNA containing the desired gene)15.
- Design primers with restriction sites for cloning; a 5′-NdeI site and a 3′-EcoRI site are recommended. Other sites can be used, if necessary; refer to the multicloning region of the selected plasmid (Figure S1-S4). If desired, include either a 5′ or 3′ encoding tag for Western blot detection.
- PCR amplify the desired gene15.
- Analyze the PCR by agarose gel electrophoresis16.
- If there is unspecific amplification, clean up the amplified gene by gel extraction. If not, use an enzyme clean-up kit.
- Quantify the DNA using a spectrophotometer by checking the absorbance at 260 nm; blank with elution buffer17.
2. Cloning Genes of Interest into a Multigene Expression System Vector, pMGX18
- Restriction digest the obtained gene of interest and the desired vector, pMGX, with NdeI and EcoRI.
NOTE: If a large amount of recircularized plasmids are obtained, allow the NdeI reaction to proceed for 1 h before adding EcoRI.- Use a 40 µL digest reaction containing 0.5-1.5 µg of DNA and 1 µL of each enzyme with 4 µL of the appropriate 10x buffer.
- For an NdeI and EcoRI digest, use EcoRI buffer and first add NdeI endonuclease. Digest for 1 h at 37 °C. Then add EcoRI endonuclease, and allow the digest to proceed for an additional hour. NdeI is sensitive to cleavages close to the end of DNA, so initial digestion by EcoRI can prevent effective digestion by NdeI.
- Electrophorese the restriction digest on agarose gel (for a 1 kb gene, use a 0.7% agarose gel at 110 V for 55 min; to select the percent of agarose gel for differing gene sizes, refer to reference16.
- Excise insert and vector bands using a clean scalpel or razor blade and place the excised gel segment into a 1.5 mL tube.
- Extract DNA from the agarose gel using a gel extraction kit according to the manufacturer's protocol.
- Ligate the gene of interest into the pMGX vector using a 3:1 insert-to-vector ratio; set up a negative ligation of digested pMGX without the insert.
- Set up a 20 µL ligation reaction containing 1 µL of T4 DNA ligase, 0.15-0.5 µg of vector DNA (~5 µL), and an appropriate amount of insert based on a 3:1 insert-to-vector ratio and the size of the gene being inserted; the pMGX backbones are between 5,312 and 5,504 bp in size. Include a negative control reaction that contains everything but the gene being inserted. Ensure that the amount of vector DNA is equivalent in the negative control ligation and the vector plus insert ligation reactions.
- Transform ligation reactions into XL1-Blue chemically competent E. coli cells and ligated plasmids pMGX-yfg1 (containing gene of interest 1), pMGX-yfg2 (containing gene of interest 2) … pMGX-yfgn (containing gene of interest n). Use aseptic technique (inside a biosafety cabinet or under a flame).
- Thaw 100 µL aliquots of chemically competent XL1-Blue E. coli on ice for 5 min and then add 5 µL of the ligation reactions. Incubate for 30 min on ice.
- Heat-shock the cells for 45 s in a water bath held at 42 °C and then add 200 µL of cold LB. Incubate them on ice for 2 min.
- Shake the cells at 37 °C, 220 rpm for 1 h and then spread plate 100 µL onto an LB-agar plate containing an appropriate selectable marker (either ampicillin or kanamycin).
- Screen the clones for positive transformants.
- Compare the colony counts on the negative control and ligation plates. A colony count ratio greater than 1:2 is desired. If there are a large number of colonies on the negative control plate, go back to Step 2.1 and review the note.
- Select 4-8 individual colonies (depending on the negative control:ligation ratio) from each ligation reaction of the different genes inserted for screening (1 n) and inoculate a 4 mL LB and the appropriate antibiotic overnight culture/individual colony. Grow cultures overnight with shaking at 37 °C and 220 rpm.
- Isolate plasmid DNA using a plasmid DNA isolation kit according to the manufacturer's protocol.
- Set up a 20 µL NdeI + EcoRI digest reaction containing 150-500 ng of DNA and 1 µL of each enzyme with 2 µL of the appropriate 10x buffer. In this case, add NdeI and EcoRI at the same time that the gene of interest will be in between the restriction sites. A negative control with the empty pMGX vector is recommended. Digest for 2 h at 37 °C in a water bath.
3. Inserting Gene 2 into the pMGX Vector Containing Gene 1, pMGX-yfg1
- Restriction digest pMGX-yfg1 with AvrII and treat with calf intestinal phosphatase (CIP).
- Use a 40-µL digest reaction containing 0.5-1.5 µg of vector DNA (~5-10 µL of isolated DNA) and 1 µL of AvrII with 4 µL of the appropriate 10x buffer. Digest for 1.5 h at 37 °C and then add 1.5 µL of CIP. Leave it at 37 °C for an additional 30 min.
- Restriction digest pMGX-yfg2 with AvrII and XbaI to liberate the gene of interest.
- Use a 40 µL digest reaction containing 0.5-1.5 µg of DNA and 1 µL of each enzyme with 4 µL of the appropriate10x buffer. Digest for 2 h at 37 °C.
- Electrophorese restriction digests on an appropriate percent (0.7%) agarose gel and excise the insert and vector bands using a clean scalpel/razor blade (refer to Steps 2.2-2.3).
- Extract the DNA using a gel extraction kit according to the manufacturer's protocol and quantify the DNA17.
- Ligate gene 2 into pMGX-yfg1 using a 3:1 insert-to-vector ratio. Set up a negative ligation of digested pMGX-yfg1 without the additional insert. Set up as above in Step 2.5.
- Transform 5 µL of the ligation reactions into XL1-Blue chemically competent E. coli cells, ligated plasmids pMGX-yfg1,2 (containing gene of interest 1 and 2), and negative pMGX-yfg1 control, as seen in Step 2.6.
- Compare the colony count on the negative control and ligation plates. A colony count ratio greater than 1:2 is desired. If there are a large number of colonies on the negative control plate, go back to Step 3.1 and review the CIP treatment.
- Select 4-8 individual colonies (depending on the negative control:ligation ratio) from the ligation reaction and inoculate 4 mL of LB plus the appropriate antibiotic per individual colony and grow overnight at 37 °C and 220 rpm.
- Isolate plasmid DNA using a plasmid DNA isolation kit according to the manufacturer's protocol and quantify the DNA17.
- Screen the effective insertion of the second gene by performing a restriction digest of pMGX-yfg1,2 with EcoRI.
- Use a 20 µL digest reaction containing 150-500 ng of DNA and 1 µL of EcoRI enzyme with 2 µL of EcoRI 10x buffer. Digest for 2 h at 37 °C.
- Electrophorese the restriction digest on an appropriate percent agarose gel; look for a band that corresponds to the size of gene 2 (see Step 2.2). A gene may insert into the vector in the undesired, reversed orientation.
4. Adding a Third Gene into the pMGX Vector Containing Genes 1 and 2, pMGX-yfg1,2
- Restriction digest pMGX-yfg1,2 with AvrII and treat with CIP, as seen in Step 3.1.
- Restriction digest pMGX-yfg3 with AvrII and XbaI, as seen in Step 3.2.
- Electrophorese the restriction digests on an appropriate percent agarose gel and excise insert and vector bands using a clean scalpel/razor blade; refer to Steps 2.2-2.3.
- Extract the DNA from the agarose gel using a gel extraction kit and quantify the DNA17.
- Ligate gene 3 into pMGX-yfg1,2 using a 3:1 insert-to-vector ratio and set up a negative ligation of digested pMGX-yfg1,2 without an additional insert, as seen in Step 3.5.
- Transform 5 µL of the ligation reactions into XL1-Blue chemically competent E. coli cells, ligated plasmid pMGX-yfg1,2,3 (containing genes of interest 1, 2, and 3), and negative pMGX-yfg1,2 control, as seen in Step 2.6.
- Compare the colony count on the negative control and ligation plates. If there are a large number of colonies on the negative control plate, go back to Step 4.1 and review the CIP treatment.
- Select 4-8 individual colonies (depending on the negative control:ligation ratio) from the ligation reaction and inoculate 4 mL of LB plus the appropriate antibiotic per individual colony; grow overnight at 37 °C and 220 rpm.
- Isolate the plasmid DNA using a plasmid DNA isolation kit according to the manufacturer's protocol.
- Screen the effective insertion of the third gene by performing a restriction digest of pMGX-yfg1,2,3 with EcoRI, as seen in Step 3.10.
- Electrophorese the restriction digest on an appropriate percent agarose gel; look for bands that correspond to the sizes of gene 2 and gene 3 (see Step 2.2). Note: gene 3 may insert into the vector in the undesired, reversed orientation. If genes 2 and 3 are the same size, another appropriate restriction digest site must be selected for screening.
NOTE: Repeat as needed for each new gene.
5. Producing Proteins of Interest Using a Multigene Expression System and Assessing Production by Western Blotting
- Transform the positive clone containing all genes of interest into chemically competent, protein-production E. coli, such as BL21-(λDE3).
- Thaw 100 µL aliquots of chemically competent BL21-(λDE3) E. coli on ice for 5 min and then add 1 µL of the positive cloned plasmid DNA; incubate for 30 min on ice.
- Heat shock the cells for 45 s in a water bath held at 42 °C and then add 200 µL of cold LB. Incubate on ice for 2 min.
- Shake the cells at 37 °C and 220 rpm for 1 h and then spread plate 100 µL onto an LB-agar plate containing the appropriate selectable marker (either ampicillin or kanamycin).
- Express the protein by isopropyl-β-D-1-thiogalactopyranoside (IPTG) induction.
- Select an isolated colony from the B21-(λDE3) transformation plate and inoculate 4 mL of LB plus the appropriate antibiotic; grow overnight, shaking at 37 °C and 220 rpm.
- Inoculate a 100 mL LB plus the appropriate antibiotic culture using 1 mL of overnight culture.
- Grow at 37 °C with shaking at 220 rpm to an OD600 of 0.6.
- Induce the culture with 100 µM IPTG and grow for 15 h at 25 °C and 220 rpm.
- Remove 1 mL of the culture and centrifuge it at 13,000 x g for 1 min; discard the supernatant.
- Lyse the cells using lysis solution as per the manufacturer's instructions and preform a Western blot of the soluble cell lysate to determine if all proteins were successfully produced19.
Access restricted. Please log in or start a trial to view this content.
Wyniki
In this study, the goal was to co-express five proteins from a single plasmid. The five-codon optimized synthetic gene fragments encoding either N- or C-terminal hexahistidine tags were purchased commercially. The synthetic genes were amplified by PCR and individually cloned into a PCR-blunt vector and sequenced. To generate the polycistronic plasmid, the five genes of interest were first cloned into a suitable pMGX plasmid, pMGX-A. Figure 2 shows PCRBlunt-
Access restricted. Please log in or start a trial to view this content.
Dyskusje
Co-expression of multiple genes is increasingly essential, particularly in characterizing and reconstituting complex, multigene metabolic pathways3,4,5. The pMGX system makes multigene co-expression in E. coli routine6,7,8 and accessible to diverse researchers. In this study, five proteins of interest were shown to be simultane...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This work was supported by the Natural Sciences and Engineering Research Council of Canada.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| Enzymes | |||
| Alkaline Phosphatase, Calf Intestinal (CIP) | New England Biolabs | M0290S | |
| AvrII | New England Biolabs | R0174S | |
| EcoRI | New England Biolabs | R0101S | |
| NdeI | New England Biolabs | R0111S | |
| XbaI | New England Biolabs | R0145S | |
| Herculase II Fusion DNA Polymerase | Agilent Technologies | 600677 | |
| T4 DNA Ligase | New England Biolabs | M0202S | |
| Name | Company | Catalog Number | Comments |
| Reagents | |||
| 1 kb DNA ladder | New England Biolabs | N3232L | |
| 4-20% Mini-PROTEAN TGX Stain-Free Protein Gels | Bio-Rad | 456-8095 | |
| 50x TAE | Fisher Thermo Scientific | BP1332-4 | |
| Agar | Fisher Thermo Scientific | BP1423-500 | |
| Agarose | Fisher Thermo Scientific | BP160-500 | |
| Ampicilin | Sigma-Alrich | A9518-5G | |
| BL21 (DE3) chemically comeptent cells | Comeptent cell prepared in house | ||
| B-PER Bacterial Protein Extraction Reagent | Fisher Thermo Scientific | PI78243 | |
| dNTP mix | Agilent Technologies | Supplied with polymerase | |
| Gel Extraction Kit | Omega | D2500-02 | E.Z.N.A Gel Extraction, supplied by VWR Cat 3: CA101318-972 |
| Glycine | Fisher Thermo Scientific | BP381-1 | |
| His Tag Antibody [HRP], mAb, Mouse | GenScript | A00612 | |
| Immobilon Western Chemiluminescent HRP Substrate | EMD Millipore | WBKLS0100 | |
| IPTG | Sigma-Alrich | 15502-10G | |
| LB | Fisher Thermo Scientific | BP1426-500 | |
| Methanol | Fisher Thermo Scientific | A411-20 | |
| Pasteurized instant skim milk powder | Local grocery store | No-name grocery store milk is adequate | |
| Nitrocellulose membrane | Amersham Protran (GE Healthcare Life Sciences) | 10600007 | Membrane PT 0.45 µm 200 mm x 4 m, supplied by VWR Cat #: CA10061-086 |
| Plasmid DNA Isolation Kit | Omega | D6943-02 | E.Z.N.A Plasmid DNA MiniKit I, supplied by VWR Cat #: CA101318-898 |
| pMGX | Boddy Lab | Request from the Boddy Lab Contact cboddy@uottawa.ca | |
| Primers | Intergrated DNA Technologies | Design primers as needed for desired gene | |
| Synthetic Gene | Life Technologies | Design and optimize as needed | |
| Thick Blot Filter Paper | Bio-Rad | 1703932 | |
| Tris base | BioShop | TRS001.1 | |
| Tween-20 | Sigma-Alrich | P9416-50ML | |
| XL1-Blue chemically competent cells | Comeptent cell prepared in house | ||
| Name | Company | Catalog Number | Comments |
| Equipment | |||
| BioSpectrometer | Eppendorf | RK-83600-07 | |
| Gel box - PAGE | Bio-Rad | 1658005 | Mini-PROTEIN Tetra Vertical Electrophoresis Cell |
| Gel Imager | Alpha Innotech | AlphaImager EC | |
| Incubator-oven | Fisher Thermo Scientific | 11-690-650D | Isotemp |
| Incubator-shaker | Fisher Thermo Scientific | SHKE6000-7 | MaxQ 6000 |
| Personna Razors | Fisher Thermo Scientific | S04615 | |
| Power Pack | Bio-Rad | S65533Q | FB300 |
| Transilluminator | VWR International | M-10E,6W | |
| Thermocylcer | Eppendorf | Z316091 | Mastercycler Personal, supplied by Sigma |
| UV Face-Shield | 18-999-4542 | ||
| Waterbath | Fisher Thermo Scientific | 15-460-2SQ | |
| Western Transfer Apparatus | Bio-Rad | 1703935 | Mini-Trans Blot Cell |
Odniesienia
- Purnick, P. E. M., Weiss, R. The second wave of synthetic biology: from modules to systems. Nat. Rev. Mol. Cell Biol. 10 (6), 410-422 (2009).
- Bieniossek, C., et al. Automated unrestricted multigene recombineering for multiprotein complex production. Nat. Methods. 6 (6), 447-450 (2009).
- Jochimsen, B., et al. Five phosphonate operon gene products as components of a multi-subunit complex of the carbon-phosphorus lyase pathway. Proc. Natl. Acad. Sci. USA. 108 (28), 11393-11398 (2011).
- Martin, V. J. J., Pitera, D. J., Withers, S. T., Newman, J. D., Keasling, J. D. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat. Biotechnol. 21 (7), 796-802 (2003).
- Ajikumar, P. K., et al. Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science. 330 (6000), 70-74 (2010).
- Boddy, C. N., Hotta, K., Tse, M. L., Watts, R. E., Khosla, C. Precursor-directed biosynthesis of epothilone in Escherichia coli. J. Am. Chem. Soc. 126 (24), 7436-7437 (2004).
- Lundgren, B. R., Boddy, C. N. Sialic acid and N-acyl sialic acid analog production by fermentation of metabolically and genetically engineered Escherichia coli. Org. Biomol. Chem. 5 (12), 1903-1909 (2007).
- Horsman, M. E., Lundgren, B. R., Boddy, C. N. N-Acetylneuraminic Acid Production in Escherichia coli Lacking N-Acetylglucosamine Catabolic Machinery. Chem. Eng. Commun. 203 (10), 1326-1335 (2016).
- Romier, C., et al. Co-expression of protein complexes in prokaryotic and eukaryotic hosts: experimental procedures, database tracking and case studies. Acta Crystallogr. D, Biol. Crystallogr. 62 (Pt 10), 1232-1242 (2006).
- Tolia, N. H., Joshua-Tor, L. Strategies for protein coexpression in Escherichia coli. Nat. Methods. 3 (1), 55-64 (2006).
- Chanda, P. K., Edris, W. A., Kennedy, J. D. A set of ligation-independent expression vectors for co-expression of proteins in Escherichia coli. Protein Expr. Purif. 47 (1), 217-224 (2006).
- Kim, K. -J., et al. Two-promoter vector is highly efficient for overproduction of protein complexes. Protein Sci. 13 (6), 1698-1703 (2004).
- Tan, S., Kern, R. C., Selleck, W. The pST44 polycistronic expression system for producing protein complexes in Escherichia coli. Protein Expr. Purif. 40 (2), 385-395 (2005).
- Chen, X., Pham, E., Truong, K. TEV protease-facilitated stoichiometric delivery of multiple genes using a single expression vector. Protein Sci. 19 (12), 2379-2388 (2010).
- Lorenz, T. C. Polymerase chain reaction: basic protocol plus troubleshooting and optimization strategies. J. Vis. Exp. (63), e3998(2012).
- Lee, P. Y., Costumbrado, J., Hsu, C. -Y., Kim, Y. H. Agarose gel electrophoresis for the separation of DNA fragments. J. Vis. Exp. (62), (2012).
- Sukumaran, S. Concentration determination of nucleic acids and proteins using the micro-volume BioSpec-nano-spectrophotometer. J. Vis. Exp. (48), (2011).
- JoVE Science Education Database. Basic Methods in Cellular and Molecular Biology. Molecular Cloning. J. Vis. Exp. , Available from: https://www.jove.com/science-education/5074/molecular-cloning (2016).
- JoVE Science Education Database. Basic Methods in Cellular and Molecular Biology. The Western Blot. J. Vis. Exp. , Available from: https://www.jove.com/science-education/5065/the-western-blot (2016).
- Laible, M., Boonrod, K. Homemade site directed mutagenesis of whole plasmids. J. Vis. Exp. (27), (2009).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone