Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Preparation of Keratin Hydrolysate from Chicken Feathers and Its Application in Cosmetics
W tym Artykule
Podsumowanie
The goal of the protocol is to prepare keratin hydrolysate from chicken feathers by alkaline-enzymatic hydrolysis and to test whether adding keratin hydrolysate into a cosmetics ointment base improves skin barrier function (heightening hydration and diminishing transepidermal water loss). Tests are conducted on men and woman volunteers.
Streszczenie
Keratin hydrolysates (KHs) are established standard components in hair cosmetics. Understanding the moisturizing effects of KH is advantageous for skin-care cosmetics. The goals of the protocol are: (1) to process chicken feathers into KH by alkaline-enzymatic hydrolysis and purify it by dialysis, and (2) to test if adding KH into an ointment base (OB) increases hydration of the skin and improves skin barrier function by diminishing transepidermal water loss (TEWL). During alkaline-enzymatic hydrolysis feathers are first incubated at a higher temperature in an alkaline environment and then, under mild conditions, hydrolyzed with proteolytic enzyme. The solution of KH is dialyzed, vacuum dried, and milled to a fine powder. Cosmetic formulations comprising from oil in water emulsion (O/W) containing 2, 4, and 6 weight% of KH (based on the weight of the OB) are prepared. Testing the moisturizing properties of KH is carried out on 10 men and 10 women at time intervals of 1, 2, 3, 4, 24, and 48 h. Tested formulations are spread at degreased volar forearm sites. The skin hydration of stratum corneum (SC) is assessed by measuring capacitance of the skin, which is one of the most world-wide used and simple methods. TEWL is based on measuring the quantity of water transported per a defined area and period of time from the skin. Both methods are fully non-invasive. KH makes for an excellent occlusive; depending on the addition of KH into OB, it brings about a 30% reduction in TEWL after application. KH also functions as a humectant, as it binds water from the lower layers of the epidermis to the SC; at the optimum KH addition in the OB, up to 19% rise in hydration in men and 22% rise in women occurs.
Wprowadzenie
Slaughterhouses, the food industry, and the tanning industry annually produce immense amounts of solid keratin by-products – wool, feathers, bristles, hooves, claws, horns, and the like. According to latest statistical data, the total live weight of chickens, turkeys, ducks, and other slaughtered poultry in the USA is 62.5 billion pounds per year1; in the EU it is approximately 28.7 billion pounds per year. Considering that feathers make up to 8.5% of the total poultry weight, the USA alone annually produces approx. 5.3 billion pounds of waste feathers2.
Keratin is a protein exhibiting high chemical resistance because it is strongly cross-linked with disulfide bridges that render its processing difficult. Obtaining soluble products requires cleaving cross-links and possibly carrying out hydrolysis of the peptide bonds3. Cleavage of the disulfide bridges may proceed through a reaction of thiol anion according to the following pattern4,5:
Sa- + –SbSc– ↔ –Sb- + –SaSc-
With a very high pH level, hydrolysis of the disulfide bridges also appears, in accordance with the pattern6
–SS- + OH- → –S- + –SOH
Under mild conditions (pH approx. 8), even sulfitolysis takes place according to the following pattern:
–SS– + HSO3- → –SH + –SSO3-
The most economical way of degrading keratin is microbial breakdown, which is characterized by mild reaction conditions during processing and high breakdown efficiency (approx. 90%)7,8. Keratinases are produced by some bacteria isolated from soil and keratin waste9. Microbial keratinases hydrolyze rigid and strongly cross-linked keratin structures10 and the resulting KH prepared is rich in soluble proteins, with no loss in essential amino acids detected in it11.
In order to incorporate a protein in cosmetic preparations (e.g., emulsions, lotions, and gels), the requirements ensure that such proteins are soluble in water, the given systems are transparent, and that re-aggregation of the peptides is avoided due to hydrophobic interactions. Therefore, a common practice is to apply hydrolysates of proteins, such as hydrolyzed collagen, elastin, and keratin. When adding hydrolysates into cosmetic emulsions, steps are taken to ensure that the hydrolysate is first dissolved in water. In some cases, it is desirable that the protein (or the hydrolysate) is soluble in alcohol or other organic solvents12.
KH is normally featured in shampoos, conditioners, lotions, and nutritive serums for hair, as well as mascaras, nail polish, and eye make-up agents. The KH effects declared usually include forming a protective film, smoothing the hair or nail structure, heightened plasticity and appearance of the treated formation, regulating the consistency of products, and encouraging the formation of foam13,14. It has also been shown that KH reduces surface tension, hence supplementation in cosmetics can facilitate reduction in the amount of emulsifier added to stabilize creams. KH limit the effects of irritation triggered by cleaning agents (surfactants) to the skin, eyes, and hair, thus reducing any potential side effects of cleaning agents on tissue (e.g., dehydration of the skin, hardness, and decreased barrier function of the skin). The high buffering capability of hydrolysates is also exploited to stabilize the pH of cosmetics; peptides of shorter length have a greater buffering effect15,16. Although KHs have become established as standard components in hair and nail cosmetics as well as being utilized in products for skin care, studies on the moisturizing effects of KH do not appear in contemporary literature.
Alkaline-enzymatic technology has been developed for processing keratin by-products into KH, and active testing is in process on the effects of a number of cosmetic additives17,18,19,20,21,22. The advantage of two-stage alkaline-enzymatic hydrolysis using microbial proteases for chicken feathers achieves high efficiency under mild reaction conditions and the quality of KH is very high in contrast to hydrolysis employed in strong acids or alkalis. In the first stage, feathers are incubated at a higher temperature in an alkaline environment, which partially disrupts the keratin structure and swells the feathers; after adjusting the pH, the feathers are hydrolyzed with a proteolytic enzyme under mild conditions in the second stage. The dialyzed KH possesses a high content of proteins.
The purposes of the method described here are processing poultry feathers into a KH through alkaline-enzymatic hydrolysis and testing the effect of moisturizing properties of KH applied to O/W cosmetic emulsion. The moisturizing properties are investigated by instrumental non-invasive methods in vivo. The most frequent methods for measuring skin hydration and barrier function of SC include measuring electrical properties of the skin (conductance or capacitance). Different methods for investigating SC hydration include near infrared multispectral imagining method (NIM), nuclear magnetic resonance spectroscopy, optical coherence tomography, or transient thermal transfer23. Barrier function of SC correlates to the TEWL of SC and it is measured by the ventilated chamber method, unventilated chamber method, and open chamber method24.
Properties of the model formulations are determined using the Multi Probe adapter MPA 5 with three types of probes. The first one, corneometer CM 825, measures skin hydration by assessing changes in the electrical capacity of the skin's surface; the measuring capacitor shows changes in capacitance of the skin surface in corneometric units. The corneometer gives only a relative assessment of skin hydration25. For TEWL, the second probe, tewameter TM 300, is used for measuring the density gradient of water evaporation (in an open chamber instrument based on Fick's diffusion law) from the skin indirectly by the two pairs of sensors (temperature and relative humidity) indicating the quantity of water being transported per a defined area and period of time (g/m2/h). This method can detect even the slightest disruption of skin barrier function26. Skin pH is one indicator of barrier and anti-microbial function of the SC27. The acidity of the skin mantle was measured by a (third) skin PH 905 probe connected to the MPA 5 station. This specially designed probe consists of a flat-topped glass electrode for full skin contact, connected to a voltmeter. The system measures potential changes due to the activity of hydrogen cations surrounding the very thin layer of semi-solid forms measured at the top of the probe. The changes in voltage are displayed as pH28.
We present experiments divided into three sections: (1) Preparation of KH from chicken feathers by two-stage alkaline-enzymatic hydrolysis and its purification by dialysis (removing salts and low-molecular fractions), (2) Preparation of cosmetic formulations containing 2, 4, and 6% KH, and (3) Testing the properties of KH by measuring skin hydration, TEWL, and skin pH. Testing was carried out on 10 women with the mean age of 27.2 years and on 10 men with the mean age of 26.2 years. The method of selecting the volunteers and the testing itself were conducted in accordance with international ethical principles of bio-medical research utilizing human subjects29; all persons gave their informed consent prior to inclusion in the study. Before testing commenced, the volunteers were asked to complete a questionnaire on their health status. The volunteers committed to avoid applying any cosmetic product to the test sites and surrounding regions during the 24 h prior to and during the test period; furthermore, they were only permitted brief evening washes with running water.
Protokół
Volunteers were recruited among employees and students of our university. The method of selecting was conducted according to "International Ethical Guidelines for Biomedical Research Involving Human Subjects. Council for International Organizations of Medical Sciences, Geneva (2002)." KH is a common cosmetic ingredient used in hair-care products (shampoos, conditioners, etc.) and hence approval from the institutional review board is not required.
1. Process Chicken Feathers into KH
- Collect chicken feathers from a poultry farm.
- Wash out any insoluble impurities and blood remnants from the chicken feathers with a sufficient excess of fresh running (cold) water. Place the feathers on a flat plate and dry overnight at 50 °C.
NOTE: The protocol can be paused here. - Grind 50 g dried feathers in a cutting mill (suitable for soft to medium-hard sample materials, and fibrous materials) into a final fineness of 1.0 mm. Alternatively, the final fineness of grinded feathers can be higher, but not more than 3.0 mm.
NOTE: The protocol can be paused here. - Degrease feathers
NOTE: The most effective and economic method of degreasing poultry feather is using a commercial lipolytic enzyme.- In a stainless steel 27-L boiler container with temperature control, mix the feathers with water preheated up to 40 ± 2 °C in a weight ratio 1:75. Add a lipolytic enzyme (activity 100 KLU/g) in a dose of 1.5 - 2.0% (related to weighed-in dry feathers) and gently stir the contents with an overhead stirrer for 5 min.
- Adjust the mixture pH to 9.0 ± 0.2, the value corresponding to the maximum activity of the lipolytic enzyme by adding 1% NaOH or 1% H3PO4 solution. Stir the mixture for 5 min with an overhead stirrer, and then check and re-adjust the pH level using a laboratory bench pH/mV meter.
- Gently stir the mixture with an overhead stirrer for 24 h at 40 ± 0.5 °C. Alternatively, incubate the mixture at 40 ± 0.5 °C, and during the first 6 h of incubation stir the contents at 1 h intervals.
- Filter the mixture through a fine sieve (100 µm size) and wash the degreased feathers with a stream of fresh running (cold) water. Dry the feathers on a flat plate at 50 °C in a drying chamber overnight.
NOTE: The protocol can be paused here.
- Perform the first stage of the chicken feather hydrolysis. Mix the feathers with 0.3% KOH water solution in a weight ratio 1:50 and gently stir with an overhead stirrer at 60 ± 0.5 °C for 24 h. The pH of the mixture decreases from approx. 12.5 at the start of incubation to approx. 11.0 at the end of incubation. After finishing the first stage of hydrolysis, adjust the pH of the mixture to the level corresponding to the maximum activity of the proteolytic enzyme with 10% H3PO4 (in this case, to a level of 9.0 ± 0.2) by adding 1% NaOH.
- Perform the second stage of chicken feather hydrolysis. Add to the mixture, the proteolytic enzyme in a dose of 5.0% (related to dry matter of quantity of feathers weighed-in at the beginning of the first stage of hydrolysis). Gently stir with an overhead stirrer at 60 ± 0.5 °C for 8 h and then heat the mixture (in the same stainless steel 27-L boiler container) to a boiling point (100 °C) and boil for 10 min to inactivate the enzyme.
- Separate the solution of KH (prepared in step 1.6) from the undissolved remnant by filtering it through low-density filter paper on a Buchner funnel with slight vacuum-pressure; alternatively, use a centrifuge.
NOTE: The protocol can be paused here for several days if a solution of KH is stored at 5 ± 1 °C. - In the plastic bucket (26 cm diameter x 26 cm height) dialyze the KH using 12 K MWCO membrane to remove small peptides and salts. Pour 400 mL of the KH solution into dialysis tubing and dialyze it against 4 L of distilled water for 80 h at room temperature; change the distilled water after 18, 36, and 60 h.
- Cast a dialyzed solution of KH on an anti-adhesive plate (e.g., silicone) on a ratio of 500 mL to 1,000 cm2 plate area, vacuum dry it on a thin film at 40 ± 0.5 °C for overnight, grind to form a fine powder, and keep it in a closed vessel in a desiccator.
NOTE: The protocol can be paused here for several months if the KH powder is stored in a dry place.
2. Prepare Cosmetic Formulations with KH
NOTE: The OB used for testing was a commercial hydrophilic O/W cream base and comprised of aqua, paraffin, paraffin liquid, cetearyl alcohol, Laureth 4, sodium hydroxide, carbomer, methylparaben, and propylparaben.
- Prepare formulations containing 2, 4, and 6% KH (in accordance with the base weight of the ointment). Weigh the amount of KH powder into a polyethylene vessel (7 cm diameter x 10 cm height) and add the OB at an amount that ensures the total weight of the formulation equals 50 g; see recipe in Table 1.
| Cosmetic formulation | Weight of ointment base [g] | Weight of keratin hydrolysate [g] | Total weight [g] | |||||
| Ointment base | 50 | 0 | 50 | |||||
| Ointment base + 2 % KH | 49 | 1 | ||||||
| Ointment base + 4 % KH | 48 | 2 | ||||||
| Ointment base + 6 % KH | 47 | 3 | ||||||
Table 1: Weight-in quantities of ointment base and keratin hydrolysate to prepare cosmetic formulations.
- Homogenize the mixture with a 3-bladed laboratory blender for 10 min at 134.16 x g and mix using a mechanical overhead stirrer. Maintain the prepared formulations at 5 ± 1 °C and warm them at room temperature for 2 h prior to use.
NOTE: Homogenizing the mixture of OB with KH can be done with a non-digital stirrer as well. On a non-digital stirrer, there are scales with the approximate speed (in rpm) as well. Gentle stirring will work the best for this step.
NOTE: The protocol can be paused here for up to 5 months if the formulations are stored at 5 ± 1 °C.
3. Test the Properties of KH by Measuring Skin Hydration, TEWL, and pH
NOTE: Perform all measurements in a conditioned room at 23 ± 2 °C and the relative humidity of 56 ± 3%.
- Place 5 strips of filter paper (size 2 x 4 cm) into the physiological solution (0.90% NaCl) and leave them for approximately 1 min in the solution.
- Apply two strips to the inner side of the right forearm, and three to the inner side of the left forearm, and fix them for 4 h with adhesive plasters. This step is to degrease the skin and eliminate individual characteristics of the skin at the site. After 4 h, remove the strips and demark the areas with a permanent pen, see Figure 1.
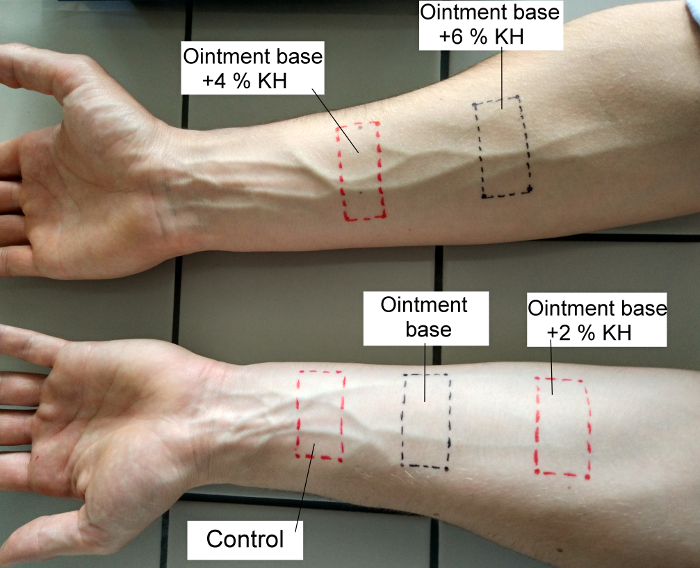
Figure 1: Method for location of test formulations on the forearm of the left and right upper limbs. Please click here to view a larger version of this figure.
- Apply 0.1 mL of the tested formulations at each spot of the degreased forearm sites using syringes and spread it over the entire marked surface. On the left forearm, do not apply anything to the first site (it is the control), apply the OB to the second site, and the OB + 2% KH to the third. Apply the OB + 4% KH and OB + 6% KH to the right arm.
- Measure each sample at each site and each interval (1, 2, 3, 4, 24, and 48 h) and take 5 readings with the skin hydration meter probe for skin hydration, 15 readings with the TEWL meter probe for skin TEWL, and 1 reading with the skin pH meter probe for skin pH. Do not allow volunteers to apply any cosmetic product to the test sites and surrounding areas during the test period; they are permitted brief evening washes with running water.
NOTE: The protocol can be paused here. - Process the resulting readings by basic numerical characteristics of the descriptive statistics, using spreadsheet software. From the 5 hydration readings measured for each sample, ignore the lowest and the highest readings, and calculate only 3 readings for the arithmetic mean and standard deviation. From the 15 TEWL readings measured for each sample, ignore the first 5, and calculate only 10 readings for arithmetic mean and standard deviation.
Wyniki
The KH prepared according to procedure presented here (see Figure 2) is yellow in color, easily soluble in water with high protein content (inorganic solids represent <2.0%); the pH of the 1.0% solution of KH is 5.3, and fulfils the requirements for cosmetic-grade hydrolysates. The yield of KH from 50 g raw material is approx. 30%. The molecular weight distribution of KH was determined by SDS-PAGE and is shown in Figure 3.
Dyskusje
The advantage of alkaline-enzymatic hydrolysis is that it can be modified according to future applications of KH. For example, in hair-care cosmetics applications where a lightly brownish color of a product is not an obstacle, a higher temperature in the hydrolysis can be applied leading to a higher yield of KH. In addition, the longer processing time during both stages of the technological procedure significantly affects the overall process efficiency – yield of KH rises to 85%.
The fin...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This article was written with support of the project IGA/FT/2017/007 of Tomas Bata University in Zlin.
Materiały
| Name | Company | Catalog Number | Comments |
| Material or chemicals | |||
| LIPEX 100T | Novozymes | LJP30020 | Lipex - enzyme produced by submerged fermentation of a genetically-modified microorganism, activity 100 KLU/g |
| Savinase Ultra 16L | Novozymes | PXN40001 | Savinase - enzyme produced by submerged fermentation of a genetically-modified microorganism, activity 16 KNPU-S/g |
| Potassium hydroxide, KOH | Sigma-Aldrich | 302510289 | Potassium hydroxide, KOH, 97,0 %, Mr 56,11 |
| Phosphoric acid solution, H3PO4 | Sigma-Aldrich | W290017 | Phosphoric acid solution, H3PO4, 85 wt. % concentration in water, Mr 98,00 |
| Sodium chloride physiological solution | Sigma-Aldrich | 52455 | Tablets of BioUltra NaCl physiological solution; 1 tablet in 1000 mL of water yields 0.9 % NaCl |
| Sodium hydroxide, NaOH | Penta s.r.o. | 40216 | Sodium hydroxide, NaOH, 97,0 %, Mr 40,00 |
| AmiFarm (Cremor base-A) | Fagron | 608425 | Hydrophilic oil in water (O/W) cream base; the composition: aqua, paraffin, paraffin liquid, cetearyl alkohol, Laureth 4, sodium hydroxide, carbomer, methylparaben, propylparaben. |
| Name | Company | Catalog Number | Comments |
| Equipment | |||
| IKA EUROSTAR POWER control-visc stirrers | IKA-labortechnik | Z404020 | Digital laboratory stirrer, for tasks up to the high viscosity range, 230V, 1/cs |
| IKA Propeller stirrer, 3-bladed | IKA-labortechnik | R 1381 | Propeller stirrer, 3-bladed, stirrer Ø: 45 mm, shaft Ø: 8 mm, shaft length: 350 mm |
| Dialysis tubing closures | Sigma-Aldrich | Z371017-10EA | Dialysis tubing closures, red, size 110 mm |
| Dialysis tubing cellulose membrane | Sigma-Aldrich | D9402-100FT | Dialysis tubing cellulose membrane, average flat width 76 mm (3.0 in.) |
| DOMO Pot with stailess, LCD | DOMO Elektronic | DO42325PC | Preserving boiler stainless steel, 2000 W, 27-L container (diameter 37 cm, height 30 cm), temperature control 30-100 ° C, operation LCD display |
| Hettich zentrifugen Universal 32 | Gemini bv | 2770 GS1R | Mid bench centrifuge, speed 18000 rpm |
| LT 3 shaking device | Fischer Scientific | 6470.0002 | Orbital shaking device |
| KERN 440-47N | Kern | 440-47N | Laboratory balance |
| KERN 770 | Kern | 770 -N | Laboratory analytical balance |
| VENTICELL 222 - Komfort | BMT, MMM Group | C 131749 | Drying oven, temperature control 30-100 ° C, air circulation control |
| Vacucell 55 - EVO | BMT, MMM Group | B 050328 | Vacuum drying oven, temperature control 30-100 ° C |
| PULVERISETTE 19 | Fritsch | 19.1030.00 | Universal cutting mill, rotor with V-cutting edges and fixed knives |
| Multi Probe Adapter System MPA 5 | Courage & Kazaka Electronic | 10225237 | MPA 5 Station - equipment for measurement hydratation, TEWL and pH |
| Skin pH-meter PH 905 probe | Courage & Kazaka Electronic | Probe to specifically measure the pH on the skin surface or the scalp | |
| Corneometer CM 825 probe | Courage & Kazaka Electronic | Probe to determine the hydration level of the skin surface (Stratum corneum). | |
| Tewameter TM 300 | Courage & Kazaka Electronic | Probe for the assessment of the transepidermal water loss (TEWL) | |
| Heidolph RZR 2020 | Heidolph | 13-225-007-03-1 | Overhead stirrer, mechanical speed setting and stepless transmission; speed range 40-2000 rpm |
| Heidolph mechanical stirrer BR 10 | Heidolph | Z336688-1EA | Blade impeller crossed stirrer |
| Fagor FS 12 | Fagor | BTT-138 | Laboratory refrigerator with freezer space |
| WTW bench pH/mV meter | WTW | Z313165 | High-performance bench pH and pH/conductivity meters for routine and high precision laboratory measurements in research or quality control laboratories |
| Container | RPC Superfos | 13-L plastic bucket, diameter 26 cm, height 26 cm | |
| Name | Company | Catalog Number | Comments |
| Software | |||
| Microsoft Office 2010 | Microsoft | ||
| C+K software | Courage and Khazaka Electronic GmbH | MPA 5 station operating software |
Odniesienia
- United States Department of Agriculture - National Agricultural Statistics Services. . Poultry Slaughter, 2016 Summary. , (2016).
- McGovern, V. Recycling poultry feathers: more bang for the cluck. Environ.Health Perspect. 108 (8), A336-A339 (2000).
- Gousterova, A., et al. Degradation of keratin and collagen containing wastes by newly isolated thermoactinomycetes or by alkaline hydrolysis. Lett. Appl. Microbiol. 40 (5), 335-340 (2005).
- Yamauchi, K., Yamauchi, A., Kusunoki, T., Khoda, A., Konishi, Y. Preparation of stable aqueous solution of keratins, and physiochemical and biodegradational properties of films. Biomed. Mater. Res. 31 (4), 439-444 (1996).
- Schrooyen, P. M. M., Dijkstra, P. J., Oberthur, R. C., Bantjes, A., Feijen, J. Partially carboxymethylated feather keratins. 2. Thermal and mechanical properties of films. J. Agric. Food Chem. 49 (1), 221-230 (2001).
- Mark, H. F., Gaylord, N. G., Bikales, N. M. . Encyclopedia of Polymer Science Technology: vol. 8: Keratin to Modacrylic Fibers. , (1968).
- Bertsch, A., Cello, N. A biotechnological process for treatment and recycling poultry feathers as a feed ingredient. Bioresour. Technol. 96 (15), 1703-1708 (2005).
- Grazziotin, A., Pimentel, F. A., de Jong, E. V., Brandelli, A. Nutritional improvement of feather protein by treatment with microbial keratinase. Animal Feed Sci. Technol. 126 (1-2), 135-144 (2006).
- Brandelli, A. Bacterial keratinases: useful enzymes for bioprocessing agroindustrial wastes and beyond. Food Bioprocess Technol. 1 (2), 105-116 (2008).
- Gusta, R., Ramnani, P. Microbial keratinases and their prospective applications: an overview. Appl.Microbiol. Biotechnol. 70 (1), 21-33 (2006).
- Vasileva-Tonkova, E., Gousterova, A., Neshev, G. Ecologically safe method for improved feather wastes biodegradation. International Biodeterior & Biodegradation. 63 (8), 1008-1012 (2009).
- Lodén, M., Barel, A. O., Paye, M., Maibach, H. I. Hydrating Substance. Handbook of Cosmetic Science and Technology. , 107-119 (2009).
- Teglia, A., Secchi, G., Goddard, E. D., Gruber, J. V. Chapter 9: Proteins in Cosmetics. Principles of Polymer Science and Technology in Cosmetics and Personal Care. , (1999).
- Magdassi, S. Delivery systems in cosmetics. Colloids and Surfaces A: Physicochem. Engin. Aspects. 123-124, 671-679 (1997).
- Dahms, G., Jung, A. Method for producing a protein hydrolysate. U.S. Patent. , (2014).
- Pons, R., Carrera, I., Erra, P., Kunieda, G., Solans, C. Novel preparation methods for highly concentrated water-in-oil emulsions. Colloids and Surfaces A: Physicochem. Engin. Aspects. 91 (3), 259-266 (1994).
- Mokrejs, P., Hrncirik, J., Janacova, D., Svoboda, P. Processing of keratin waste of meat industry. Asian J. Chem. 24 (4), 1489-1494 (2012).
- Mokrejs, P., Svoboda, P., Hrncirik, J. Processing poultry feathers into keratin hydrolysate through alkaline-enzymatic hydrolysis. Waste Manage. Res. 29 (3), 260-267 (2011).
- Mokrejs, P., Krejci, O., Svoboda, P. Producing keratin hydrolysates from sheep wool. Orient. J. Chem. 27 (4), 1303-1309 (2011).
- Mokrejs, P., Krejci, O., Svoboda, P., Vasek, V. Modeling technological conditions for breakdown of waste sheep wool. Rasayan J. Chem. 4 (4), 728-735 (2011).
- Polaskova, J., Pavlackova, J., Vltavska, P., Mokrejs, P., Janis, R. Moisturizing effect of topical cosmetic products applied to dry skin. J. Cosmet. Sci. 64 (5), 329-340 (2013).
- Polaskova, J., Pavlackova, J., Egner, P. Effect of vehicle on the performance of active moisturizing substances. Skin Res. Technol. 21 (4), 403-412 (2015).
- Verdier-Sévrain, S., Bonté, F. Skin hydration: a review on its molecular mechanisms. J. Cosmet. Dermatol. 6 (2), 75-82 (2007).
- Darlenski, R., Sassning, S., Tsankov, N., Fluhr, J. W. Non-invasive in vivo methods for investigation of the skin barrier physical properties. Eur. J. Pharm. Biopharm. 72 (2), 295-303 (2009).
- Berardesca, E. EEMCO guidance for assessment of stratum corneum hydration: electrical methods. Skin Res. Technol. 3 (2), 126-132 (1997).
- Rogiers, V. EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Skin Pharmacol. Appl. Skin Physiol. 14 (2), 117-128 (2001).
- Ali, S. M., Yosipovitch, G. Skin pH: from basic science to basic skin care. Acta Derm. Venereol. 93 (3), 261-267 (2013).
- Agache, P., Humbert, P. . Measuring the Skin. , (2004).
- Council for International Organizations of Medical Sciences. . International Ethical Guidelines for Biomedical Research Involving Human Subjects. , (2002).
- Ruland, J. K. Transdermal permeability and skin accumulation of amino acids. Int. J. Pharm. 72 (2), 149-155 (1991).
- Draelos, Z. D. Therapeutic moisturizers. Dermatol. Clin. 18 (4), 597-607 (2000).
- Courage and Khazaka Electronic GmbH, Technical Charges. . Information and Operating Instructions for the Multi probe Adapter MPA and its Probe. , (2013).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone