Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
One-pot Microwave-assisted Conversion of Anomeric Nitrate-esters to Trichloroacetimidates
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
A 2-azido-1-nitrate-ester can be converted to the corresponding 2-azido-1-trichloroacetimidate in a one-pot procedure. The goal of the manuscript is to demonstrate utility of the microwave reactor in carbohydrate synthesis.
Streszczenie
The goal of the following procedure is to provide a demonstration of the one-pot conversion of a 2-azido-1-nitrate-ester to a trichloroacetimidate glycosyl donor. Following azido-nitration of a glycal, the product 2-azido-1-nitrate ester can be hydrolyzed under microwave-assisted irradiation. This transformation is usually achieved using strongly nucleophilic reagents and extended reaction times. Microwave irradiation induces hydrolysis, in the absence of reagents, with short reaction times. Following denitration, the intermediate anomeric alcohol is converted, in the same pot, to the corresponding 2-azido-1-trichloroacetimidate.
Wprowadzenie
Due to their ubiquity in molecular biology, carbohydrates have been longstanding targets for chemical synthesis.1,2,3 At the core of any successful synthetic campaign is the correct deployment of glycosylation reactions to build the oligosaccharide chain.4,5,6,7,8,9,10,11,12 Not surprisingly, there are a large number of methods to install glycosidic bonds.13,14 The Koenigs-Knorr method is one of the earliest known procedures and involves coupling a glycosyl chloride or bromide with an alcoholic component, usually under heavy metal (mercury or silver) activation.15 Related glycosyl fluorides were first introduced as donors in 1981 by the Mukaiyama group and have found widespread application due to their increased thermal and chemical stability.16 On the opposite end of the reactivity spectrum are glycosyl iodides, which are far more reactive than the other halides. Increased reactivity is accompanied by increased stereocontrol, particularly when forming α-linked oligosaccharides.17 In addition to "haloglycosides", thioglycosides have found wide utility, in part, due to their ease of formation, stability to a multitude of reaction conditions, and activation with electrophilic reagents.18
The methods described above focus on converting an anomeric alcohol to a "non-oxygen" containing, latent leaving group that is activated and ultimately displaced by an alcohol from an acceptor molecule. Anomeric oxygen activation as described by the Schmidt school, focuses on converting the C1 oxygen itself, to a leaving group.19 This method is the most powerful and widely used in chemical glycosylation reactions. Trichloroacetimidate donors are readily prepared from a reducing sugar and trichloroacetonitrile in the presence of a base such as potassium carbonate (K2CO3) or 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU). These species are then activated using Lewis acids.20
Recently, we have reported that 2-azido-1-trichloroacetimidate donors can be directly prepared from glycals. The process involves a two reaction, one-pot procedure from 2-azido-1-nitrate esters.21 This detailed protocol is intended to assist practitioners in successfully completing the transformation in high yield. Of particular interest is the first step of the sequence, which focuses on thermal denitration under microwave- assisted heating. We also hope to provide a visual tutorial on employing microwave reactors in organic synthesis.
Protokół
1. Representative Microwave-Assisted Denitration
- Place the azido nitrate ester (1.0 equiv., 0.2 mmol) in an 8 mL microwave reaction vial. The scale of the reaction can be increased to several mmol without any adverse effect on reaction progress.
- Dissolve the azido-nitrate ester in 20% aq. acetone (0.1 M, 2.0 mL). Add pyridine (5.0 equiv., 0.08 mL, 1.0 mmol) to the reaction vessel. Cap the microwave irradiation vial and place the reaction vessel in a microwave reactor cavity.
- Irradiate the solution at 120 °C for 15 min with stirring and with a fixed hold time. The hold time represents how long the irradiation will occur at the designated temperature and resultant pressure. Heat all reactions to the reported temperature over a 2-minute ramping period. Monitor the temperature by a builtin IR sensor.
- After 15 min, analyze the reaction mixture using thin layer chromatography (TLC) to confirm consumption of the starting material. Use 1:1 ethyl acetate/hexanes as the eluent.
- Visualize the TLC plate using ceric ammonium molybate stain. The Rf of the reactant and product will vary, but the reducing alcohol is generally 0.05 to 0.1 lower Rf than the reactant.
2. Formation of the trichloroacetimidate
- Following complete consumption of the starting material, evaporate the solvent to a reduced volume using an airline. Then, dilute with (dichloromethane) CH2Cl2 (1.0 mL) and use a syringe to remove the water layer. Once the water layer is removed, cool the reaction mixture to 0 °C using an ice-water bath.
- Next, add DBU (10 eq, 0.3 mL, 1.9 mmol) and 2,2,2-trichloroacetonitrile (50 eq, 1.0 mL, 10 mmol) to the reaction vessel. Both reagents are added in excess and a minimum of 1 equivalent of base and 1 equivalent of 2,2,2-trichloroacetonitrile are needed.
- Allow the reaction mixture to stir while warming to ambient temperature. Monitor the reaction by TLC to confirm consumption of the starting material.
- Use 1:1 ethyl acetate/hexanes as the eluent. Visualize the TLC plate using ceric ammonium molybate stain. The Rf of the reactant and product will vary.
- After complete consumption of starting material, transfer the reaction mixture to a recovery flask and concentrate the mixture in vacuo at 30 °C. Evaporation of solvent will provide a crude pale yellow to brown oil.
- Purify the crude product by silica gel column chromatography with a 1.5 cm chromatography column and 1:4 ethyl acetate/hexanes as eluent. The physical form of the imidate will vary from molecule to molecule.
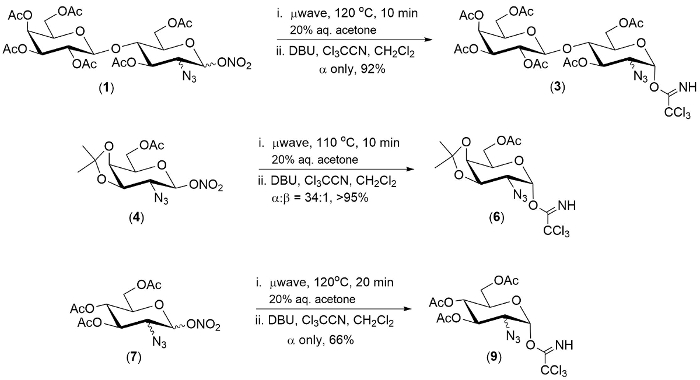
Figure 1. Representative examples of the one-pot conversion of 2-azido-1-nitrate esters to 2-azido-1-trichloroimidates. Please click here to view a larger version of this figure.
Wyniki
The technology described herein was demonstrated on a pool of three 2-azido-1-nitrate esters. In each case the first step of the reaction was complete within 20 minutes.
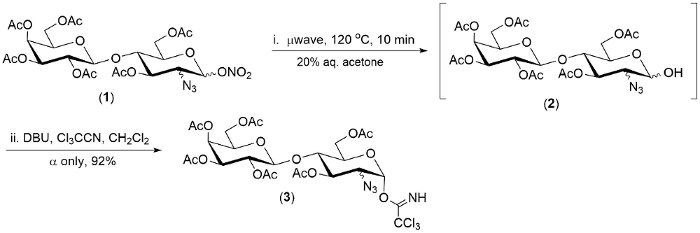
Figure 2. Representative example of hydrolysis (1 ->2), and one-pot conversion of 2-azido-1-nitrate ester of
Dyskusje
The protocol described in this tutorial provides a method to convert nitrate esters to useful, reactive functionality. In a broader sense, employing a microwave reactor to complete specific maneuvers over the course of a carbohydrate synthesis has the potential to make difficult transformations facile and routine. Our goal in this tutorial is to demonstrate how to handle carbohydrates in the context of microwave irradiation.
In the case of the parent reaction, previous efforts to achieve denit...
Ujawnienia
The authors have no competing financial interests.
Podziękowania
The authors would like to acknowledge Vanderbilt University and the Institute of Chemical Biology for financial support. Mr. Berkley Ellis and Prof. John McLean are acknowledged for High-Resolution Mass Spectral Analysis.
Materiały
| Name | Company | Catalog Number | Comments |
| 230 400 mesh silica gel | SiliCycle Inc | R10030B | |
| TLC plates | SiliCycle Inc | TLG-R10014B-527 | |
| Ceric ammonium molybdate | Sigma-Aldrich | A1343 | |
| Solvent Still | Mbraun | MB-SPS-800 | |
| Infared spectrometer | Thermo | Thermo Electron IR100 | |
| Nuclear Magnetic Resonance | Bruker | 400, 600 MHz | |
| LC/MS | Thermo/Dionex | Single quad, ESI | |
| HRMS | Agilent | Synapt G2 S HDMS | |
| Microwave reactor | Anton Parr | Anton Parr G10 Monowave 200 | |
| DBU | Sigma-Aldrich | 139009 | |
| CCl3CN | Sigma-Aldrich | T53805 | |
| Pyridine | Sigma-Aldrich | 270970 | |
| Acetone | Fisher Scientific | A18-20 | Tech. grade |
| Phase separator | Biotage | 120-1901-A | |
| Rotary evaporator | Buchi | R-100 |
Odniesienia
- Nicolaou, K. C., Mitchell, H. J. Adventures in Carbohydrate Chemistry: New Synthetic Technologies, Chemical Synthesis, Molecular Design, and Chemical Biology A list of abbreviations can be found at the end of this article. Telemachos Charalambous was an inspiring teacher at the Pancyprian Gymnasium, Nicosia, Cyprus. Angew. Chem. Int. Ed. Engl. 40 (9), 1576-1624 (2001).
- Danishefsky, S. J., Allen, J. R. From the laboratory to the clinic: A retrospective on fully synthetic carbohydrate-based anticancer vaccines. Angew. Chem. Int. Ed. Engl. 39 (5), 836-863 (2000).
- Nicolaou, K. C., Hale, C. R. H., Nilewski, C., Ioannidou, H. A. Constructing molecular complexity and diversity: total synthesis of natural products of biological and medicinal importance. Chemical Society Reviews. 41 (15), 5185-5238 (2012).
- Zhu, X., Schmidt, R. R. New principles for glycoside-bond formation. Angew. Chem. Int. Ed. Engl. 48 (11), 1900-1934 (2009).
- Danishefsky, S. J., Bilodeau, M. T. Glycals in organic synthesis: The evolution of comprehensive strategies for the assembly of oligosaccharides and glycoconjugates of biological consequence. Angew. Chem. Int. Ed. Engl. 35 (13-14), 1380-1419 (1996).
- Bongat, A. F., Demchenko, A. V. Recent trends in the synthesis of O-glycosides of 2-amino-2-deoxysugars. Carbohydr. Res. 342 (3-4), 374-406 (2007).
- Feizi, T., Fazio, F., Chai, W. C., Wong, C. H. Carbohydrate microarrays - a new set of technologies at the frontiers of glycomics. Curr. Opin. Struct. Biol. 13 (5), 637-645 (2003).
- Palmacci, E. R., Plante, O. J., Seeberger, P. H. Oligosaccharide synthesis in solution and on solid support with glycosyl phosphates. Eur. J. Org. Chem. (4), 595-606 (2002).
- Stallforth, P., Lepenies, B., Adibekian, A., Seeberger, P. H. 2009 Claude S. Hudson Award in Carbohydrate Chemistry. Carbohydrates: a frontier in medicinal chemistry. J. Med. Chem. 52 (18), 5561-5577 (2009).
- Danishefsky, S. J., Mcclure, K. F., Randolph, J. T., Ruggeri, R. B. A Strategy for the Solid-Phase Synthesis of Oligosaccharides. Science. 260 (5112), 1307-1309 (1993).
- Demchenko, A. V. Stereoselective chemical 1,2-cis O-glycosylation: From 'sugar ray' to modern techniques of the 21st century. Synlett. (9), 1225-1240 (2003).
- Fraserreid, B., Wu, Z. F., Udodong, U. E., Ottosson, H. Armed-Disarmed Effects in Glycosyl Donors - Rationalization and Sidetracking. J. Org. Chem. 55 (25), 6068-6070 (1990).
- Bohe, L., Crich, D. A propos of glycosyl cations and the mechanism of chemical glycosylation; the current state of the art. Carbohydr. Res. 403, 48-59 (2015).
- Toshima, K., Tatsuta, K. Recent Progress in O-Glycosylation Methods and Its Application to Natural-Products Synthesis. Chem. Rev. 93 (4), 1503-1531 (1993).
- Koenigs, W., Knorr, E. Ueber einige Derivate des Traubenzuckers und der Galactose. Chem. Ber. 34 (1), 957-981 (1901).
- Mukaiyama, T., Murai, Y., Shoda, S. An Efficient Method for Glucosylation of Hydroxy Compounds Using Glucopyranosyl Fluoride. Chem. Lett. (3), 431-432 (1981).
- Meloncelli, P. J., Martin, A. D., Lowary, T. L. Glycosyl iodides. History and recent advances. Carbohydrate Research. 344 (9), 1110-1122 (2009).
- Lian, G., Zhang, X., Yu, B. Thioglycosides in carbohydrate research. Carbohydr. Res. 403, 13-22 (2015).
- Schmidt, R. R., Kinzy, W. Anomeric-Oxygen Activation for Glycoside Synthesis - the Trichloroacetimidate Method. Advances in Carbohydrate Chemistry and Biochemistry. 50, 21-123 (1994).
- Schmidt, R. R., Toepfer, A. Glycosylation with highly reactive glycosyl donors: efficiency of the inverse procedure. Tetrahedron Lett. 32 (28), 3353-3356 (1991).
- Keith, D. J., Townsend, S. D. Direct, microwave-assisted substitution of anomeric nitrate-esters. Carbohydr. Res. 442, 20-24 (2017).
- Bukowski, R., et al. Synthesis and Conformational Analysis of the T-Antigen Disaccharide(B-D-Gal-(1->3)-a-D-GalNAc-OMe). Eur. J. Org. Chem. 14, 2697-2705 (2001).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone