Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
An Experimental Model of Diet-Induced Metabolic Syndrome in Rabbit: Methodological Considerations, Development, and Assessment
W tym Artykule
Podsumowanie
We describe methods to develop an experimental model of diet-induced metabolic syndrome (MetS) in rabbits using a high-fat, high-sucrose diet. Animals developed central obesity, mild hypertension, pre-diabetes, and dyslipidemia, thus reproducing the main components of human MetS. This chronic model will allow acquisition of knowledge underlying mechanisms of disease progression.
Streszczenie
In recent years, obesity and metabolic syndrome (MetS) have become a growing problem for public health and clinical practice, given their increased prevalence due to the rise of sedentary lifestyles and unhealthy eating habits. Thanks to animal models, basic research can investigate the mechanisms underlying pathological processes such as MetS. Here, we describe the methods used to develop an experimental rabbit model of diet-induced MetS and its assessment. After a period of acclimation, animals are fed a high-fat (10% hydrogenated coconut oil and 5% lard), high-sucrose (15% sucrose dissolved in water) diet for 28 weeks. During this period, several experimental procedures were performed to evaluate the different components of MetS: morphological and blood pressure measurements, glucose tolerance determination, and the analysis of several plasma markers. At the end of the experimental period, animals developed central obesity, mild hypertension, pre-diabetes, and dyslipidemia with low HDL, high LDL, and an increase of triglyceride (TG) levels, thus reproducing the main components of human MetS. This chronic model allows new perspectives for understanding the underlying mechanisms in the progression of the disease, the detection of preclinical and clinical markers that allow the identification of patients at risk, or even the testing of new therapeutic approaches for the treatment of this complex pathology.
Wprowadzenie
Obesity and metabolic syndrome (MetS) have become a growing problem for public health and clinical practice, given their increased prevalence due to the rise of sedentary lifestyles and unhealthy eating habits1. There are several definitions of MetS, but most of them describe it as a cluster of cardiovascular and metabolic alterations such as abdominal obesity, reduced HDL and elevated LDL cholesterol, elevated triglycerides, glucose intolerance, and hypertension2,3,4. Diagnosis requires that three out of these five criteria are present.
Owing to animal models, basic research has been able to investigate the mechanisms underlying pathological processes such as MetS. Several animal models have been used, but it is of crucial importance that the model of choice reproduces the main clinical manifestations of the human pathology (Figure 1). With this aim, animal models considered similar to humans, mainly canine and swine, have been developed (see Verkest5 and Zhang & Lerman6 for review). However, canine models do not show all the components of MetS, given that the development of atherosclerosis or hyperglycemia in dogs by means of the diet is questionable5. Swine models present the most anatomical and physiological similarity with humans, and thus offer significant predictive power for elucidating the mechanisms underlying MetS, but their maintenance and the complexity of the experimental procedures make the use of this model very labor intensive and costly6.
On the other hand, rodent models (mouse and rat), diet-induced spontaneous and transgenic, have been extensively used in the literature for the study of obesity, hypertension, and MetS, and its pathological consequences in different organs and systems (see Wong et al.7 for review). Although the use of these models is more affordable than canine or swine, they have important drawbacks. Indeed, depending on the strain, animals develop some components of MetS, whereas others such as hypertension, hyperglycemia, and hyperinsulinemia are absent7. Furthermore, one of the main components of MetS, obesity, in some genetically modified strains, does not only depend on factors associated with the diet, rather it has been shown that some animals become obese with normal or even reduced food intake8. Finally, mice and rats show a natural deficiency in cholesteryl ester transfer protein (CETP) and use HDL as the major means of cholesterol transport, which makes them relatively resistant to the development of atherosclerosis. This is an important difference in lipid metabolism with humans, who express CETP and transport their cholesterol mainly in LDL9.
Conversely, the laboratory rabbit represents an intermediate stage between the larger animal and rodent experimental models. Thus, the rabbit can be easily submitted to different types of protocols with minimal requirements of personnel and maintenance, being more easily handled in experimental procedures than larger animal models. Furthermore, it has been reported that rabbits fed with a high-fat diet have similar hemodynamic and neurohumoral changes as obese humans8,10,11. Of note, regarding lipid metabolism, the rabbit has abundant CETP in plasma and their lipoprotein profile is LDL-rich12, which is also similar to humans. Additionally, rabbits develop hyperlipidemia quite rapidly given that, as herbivores, they are very sensitive to dietary fat13.
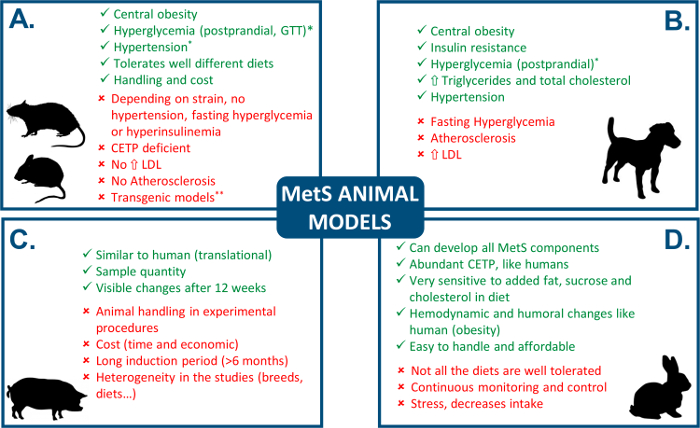
Figure 1: Comparison of MetS animal models. See Verkest5, Zhang and Lerman6, and Wong et al.7 for review. " " indicates an advantage and "
" indicates an advantage and " " indicates a disadvantage. *controversial, depends on the study, **as pointed out by Carroll et al.8, some genetically modified strains become obese independently of food intake. CEPT: cholesteryl ester transfer protein. GTT: glucose tolerance test. Please click here to view a larger version of this figure.
" indicates a disadvantage. *controversial, depends on the study, **as pointed out by Carroll et al.8, some genetically modified strains become obese independently of food intake. CEPT: cholesteryl ester transfer protein. GTT: glucose tolerance test. Please click here to view a larger version of this figure.
In order to elucidate the basic mechanisms underlying the pathological remodeling produced by MetS in the different organs and systems, and to gain understanding of this complex pathology, the choice of an experimental model that reproduces the main components of human MetS is essential. The rabbit can provide many advantages given its similarity with human physiology and the affordability of use in chronic protocols and measurements. In this line, few diet-induced rabbit models using high-fat and high-sucrose diet have been used14,15,16,17,18,19 (Table 1), and a characterization of the different components of MetS is of great importance when relating a phenotype with organ remodeling. Thus, this article's main objective is to describe the methods to develop a model of diet-induced MetS in rabbits that allows the study of its pathophysiology and impact in organ remodeling.
| Study | Diet | Duration | Breed | MetS components | |||
| Ob | HT | HG | Dl | ||||
| Yin et al. (2002)14 | · 10% fat | 24 weeks | · Male NZW |  | - |  |  |
| · 37% sucrose | · 2 kg | ||||||
| Zhao et al. (2007)15 | · 10% fat | 36 weeks | · Male JW |  |  |  |  |
| · 30% sucrose | · 16 weeks | ||||||
| Helfestein et al. (2011)16 | · 10% fat | 24 weeks | · Male NZW |  | - |  |  |
| · 40% sucrose | · 12 weeks | ||||||
| · 0.5-0.1 cholesterol | |||||||
| Ning et al. (2015)17 | · 10% fat | 8-16 weeks | · Male WHHL |  | - |  |  |
| · 30% fructose* | · 12 weeks | ||||||
| Liu et al. (2016)18 | · 10% fat | 48 weeks | · Male NZW |  | - |  |  |
| · 30% sucrose | · 12 weeks | ||||||
| Arias-Mutis et al. (2017)19 | · 15% fat | 28 weeks | · Male NZW |  |  |  |  |
Table 1: Diet-induced MetS rabbit models using high-fat, high-sucrose diet. The symbol " " indicates absence, "
" indicates absence, " " presence, and "-" not evaluated. *restricted. WHHL, Watanabe heritable hiperlipidemic rabbits. JW, Japanese white rabbits. Ob, obesity. HT, hypertension. HG, hyperglycemia. Dl, dyslipidemia.
" presence, and "-" not evaluated. *restricted. WHHL, Watanabe heritable hiperlipidemic rabbits. JW, Japanese white rabbits. Ob, obesity. HT, hypertension. HG, hyperglycemia. Dl, dyslipidemia.
Protokół
Animal care and the experimental protocols used in this study complied with EU directive 2010/63 on the protection of animals used for scientific purposes, and were approved by the Institutional Animal Care and Use Committee (2015/VSC/PEA/00049).
NOTE: The protocol consists of the chronic administration of a high-fat, high-sucrose diet for 28 weeks, and the assessment of the main components of MetS. We used 11 adult male New Zealand White (NZW) rabbits weighing 4.39 ± 0.14 (s.d.) kg, which were 20 - 22 weeks old at the beginning of the experimental protocol. They were housed in a room with humidity (50 ± 5%) and temperature (20 ± 1.5 °C) controlled conditions with a 12-h light cycle. The words "chow" and "diet" may be used interchangeably in the protocol steps.
1. Diet Administration
- Obtain or prepare diets
- Obtain a commercially available high fat diet with added hydrogenated coconut oil (10%) and lard (5%)19. This diet will provide 3.7 kcal·g-1.
- Prepare 5 to 15% sucrose solutions by dissolving the appropriate amounts of sucrose in sterilized water (e.g., use 300 g sucrose in 2 L stock for a 15% sucrose solution). A 15% solution will provide 0.6 kcal·mL-1.
- Obtain a commercially available control diet19, which provides 2.7 kcal·g-1.
- Acclimate the animals for 4 weeks
- Feed each animal in the control group 120 g of control diet daily. Provide water ad libitum.
- Feed animals in MetS group 250 g chow starting with a 50% control and 50% high-fat chow, increasing progressively to 100% high-fat chow by the end of week 4.
NOTE: The aim would be to achieve: (i) 35% control and 65% high-fat chow by the end of week 1; (ii) 25% control and 75% high-fat chow by the end of week 2; (iii) 15% control and 85% high-fat chow by the end of week 3. (iv) 100% high-fat chow by the end of week 4. - Give animals in the MetS group water with 5% sucrose at the start, and increase sucrose concentration to 15% by the end of the 4th week.
- Register the daily intake of chow and sucrose solution to calculate caloric intake as per values provided in 1.1.1. and 1.1.2.
- Induce MetS (28 weeks)
- Feed each animal in control group 120 g of control chow and water ad libitum daily.
- Feed the animals in the MetS group 250 g of high-fat chow and 15% sucrose in water. Replace chow daily and the sucrose solution every third day.
- Weigh the remaining chow and water daily to estimate daily intake.
2. Morphological Assessment
- Measure animal bodyweight on a weekly basis.
- Measure height, length, abdominal contour, and tibial length, and estimate BMI before administration of the experimental diet and at weeks 14 and 28 in anesthetized animals.
- Cannulate right ear marginal vein with a sterile disposable catheter (18 - 22 G) and inject propofol (8 mgkg-1) followed by 1.5 mL of 0.9% NaCl solution. In the anesthetized rabbit, perform the measurements listed in the subsequent steps.
- Measure height and length. Using measuring tape, measure and record the distance from the nose to the heel in lateral decubitus position (length). In the same position, take the distance from the acromion in the shoulder to the tip of the paw (height).
- Calculate Body mass index (BMI)20 as bodyweight (kg) · [body length (m) × height (m)]-1.
- Place the measuring tape gently around the abdominal contour and take a measurement with the animal in supine position.
- Measure tibial length from the lower part of the knee joint to the insertion of Achilles tendon.
3. Fasting Glycemia and Intravenous Glucose Tolerance Test (IVGTT)
NOTE: It is advisable to start the procedures the same time of day (i.e., 2 - 3 PM).
- Prepare a glucose stock solution (60%) with 60 g glucose in 100 mL of 0.9% NaCl solution.
- Fast the animal for 7 h (removing food and maintaining water), then place the conscious rabbit in a restrainer in the prone position. Prepare the glucose meter (insert a new strip into the meter), and take the first sample from the left ear marginal vein using a lancet to get a drop of blood. Then touch the blood drop with the test strip and measure blood glucose levels using the glucose meter to determine fasting glycemia.
- Cannulate right ear marginal vein with a disposable catheter (18 - 22 G) and inject a bolus of a 60% glucose solution (0.6 g·kg-1).
NOTE: To prepare the bolus, add 1 mL/kg of the glucose stock. - Take blood samples using the lancet (one drop of blood) at 15, 30, 60, 90, 120, and 180 min after glucose injection and analyze them with the glucose meter as in 3.2.
- Remove the disposable catheter and pinch the site of catheter insertion with a gauze. Once blood has coagulated, remove the gauze and check the status of the animal.
4. Blood Pressure
- Prepare the acquisition system including a pressure transducer, a 10-mL syringe with 0.9% NaCl, a three-way stopcock, an amplifier, and a PC/laptop with the acquisition software (for blood pressure recording).
- Set up the equipment. First, place the three-way stopcock and the syringe in the pressure transducer, between the transducer and the catheter, and connect the pressure transducer to the amplifier. Then connect the amplifier to the PC/laptop.
- Perform the pressure transducer calibration according to the manufacturer's recommendations.
- Place the conscious animal in a rabbit restrainer in the prone position. Warm up the ear before cannulation, then topically apply a local anesthetic (2.5% lidocaine/prilocaine) in the ear around the site of insertion. Gently tap the area where the vascular package runs to easily identify the artery. Then insert a sterile catheter (18 - 22 G) in the left ear central artery. Loosen the restraints and allow the animal to stay quiet for 30 min.
- Record blood pressure continuously for 20 min directly from the arterial catheter, placing the pressure transducer positioned next to the animal at the heart level (sampling frequency: 1 KHz, see Figure 5B).
NOTE: To keep the blood pressure (BP) recording free from blood coagulation interference (BP signal loses amplitude or disappears), an NaCl (0.9%) injection should be made. Using the three-way stopcock, close the circuit that goes from the transducer to the catheter, open the circuit that goes from the syringe to the catheter, and inject 1 - 2 mL. This will remove blood clots that may form in the catheter. Then, open the circuit between the transducer and the catheter, and continue the recording once the signal has been recovered. - Once the recording is finished, remove the catheter and pinch with a gauze in the site of catheter insertion to stop blood loss. Once the blood has coagulated, remove the gauze and check the status of the animal.
5. Plasma Measurements
NOTE: It is advisable to start the procedures the same time of day (i.e., 2 - 3 PM).
- Fast the animal for 7 h (removing food and maintaining water), then place the conscious animal in a restrainer in the prone position and insert a sterile 21 G needle in the left ear marginal vein. Once blood begins to drip, discard the first drop and collect the blood samples in EDTA tubes up to the level indicated in the tube. Store the samples on ice.
- Centrifuge blood samples at 1,500 x g, 15 min, 4 °C. After centrifugation, suction the plasma using a pipette and prepare aliquots of 250 µL.
- Analyze the fresh samples immediately. Basic control parameters are as follows: triglycerides, total cholesterol, HDL, and LDL cholesterol.
NOTE: Samples not freshly analyzed should be stored immediately in a -80 °C freezer. If interested in analyzing blood glucose from plasma samples, the blood glucose test should use tubes with Fluoride Oxalate instead of EDTA.
Wyniki
MetS represents a cluster of metabolic and cardiovascular abnormalities whose study can be facilitated by the use of experimental models. Indeed, to elucidate the mechanisms underlying the pathological remodeling produced by MetS, the choice of an experimental model that appropriately resembles the human condition and is suitable for research is of crucial importance. Here, we present the methods to induce MetS in rabbit using a diet high in saturated fat and sucrose, and a detailed chara...
Dyskusje
The establishment of an appropriate experimental model can provide a more consistent and reliable method to study the development of MetS, and it is also necessary to understand the basic mechanisms that underlie the organs and systems remodeling. Here, we describe the methods used to develop a relevant experimental model of diet-induced MetS and how to assess the main components of this cluster of metabolic and cardiovascular abnormalities that characterize this model: central obesity, hypertension, glucose intolerance,...
Ujawnienia
The authors declare that they have no competing financial interests.
Podziękowania
This work was supported by Generalitat Valenciana (GV2015-062), Universitat de València (UV-INV-PRECOMP14-206372) to MZ, Generalitat Valenciana (PROMETEOII/2014/037) and Instituto de Salud Carlos III-FEDER funds (CIBERCV CB16/11/0486) to FJC.
Materiały
| Name | Company | Catalog Number | Comments |
| Veterinary scale | SOEHNLE | 7858 | Scale https://www.soehnle-professional.com/en/productgroup/details/103/veterinary-scale |
| Shovel for aluminum feed | COPELE | 10308 | Shovel for aluminum feed http://copele.com/es/herramientas/48-pala-para-pienso-de-aluminio.html |
| Balance | PCE Ibérica | PCE-TB 15 | Balance http://www.pce-iberica.es/medidor-detalles-tecnicos/balanzas/balanza-compacta-pce-bdm.htm |
| Strainer (20 cm diam.) | ZWILLING | 39643-020-0 | Strainer https://es.zwilling-shop.com/Menaje-del-hogar/Menaje-de-cocina/Menaje-especial/Accesorios/Colador-20-cm-ZWILLING-39643-020-0.html |
| Bowl | ZWILLING | 40850-751-0 | Scale https://www.soehnle-professional.com/en/productgroup/details/103/veterinary-scale |
| Funnel | BT Ingenieros | not available | Funnel http://www.bt-ingenieros.com/fluidos-y-combustibles/961-juego-de-4-embudos-de-plastico.html?gclid=EAIaIQobChMIuInui_y-1QIVASjTCh28Zwf-EAQYBSABEgK7xPD_BwE |
| Introcan Certo 22G blue | B Braun | 4251318 | Peripheral intravenous catheter http://www.bbraun-vetcare.es/producto/introcan- |
| Propofol Lipuro 10 mg/ml vial 20 ml | B Braun | 3544761VET | General intravenous anesthetic http://www.bbraun-vetcare.es/producto/propofol-lipuro-1- |
| FisioVet serum solution 500ml | B Braun | 472779 | Scale https://www.soehnle-professional.com/en/productgroup/details/103/veterinary-scale |
| Askina Film Vet 1,25cm x 5m | B Braun | OCT13501 | Plastic Plaster http://www.bbraun-vetcare.es/producto/askina-film-vet |
| Askina Film Vet 2,50cm x 5m | B Braun | OCT13502 | Plastic Plaster http://www.bbraun-vetcare.es/producto/askina-film-vet |
| Injekt siringe 10ml luer | B Braun | 4606108V | Injection-aspiration syringe of two single-use bodies http://www.bbraun-vetcare.es/producto/injekt- |
| Seca 201 | seca | seca 201 | Ergonomic tape for measuring perimeters https://www.seca.com/es_es/productos/todos-los-productos/detalles-del-producto/seca201.html#referred |
| Sterican 21Gx1" - 0,8x25mm verde | B Braun | 4657543 | Single Use Hypodermic Needle http://www.bbraun-vetcare.es/producto/agujas-hipodermicas-sterican- |
| CONTOURNEXT-Meter | BAYER | 84413470 | Blood glucose analysis system http://www.contournextstore.com/en/contour-next-meter-2 |
| CONTOUR NEXT test strips | BAYER | 83624788 | Blood glucose test strips http://www.contournextstore.com/en/contour-next-test-strips-100-ct-package |
| MICROLET NEXT LANCING DEVICE | BAYER | 6702 | Lancing device http://www.contournextstore.com/en/new-microlet-next-lancing-device |
| MICROLET 2 Colored Lancets | BAYER | 81264857 | Ultra-thin sterile lancet for capillary puncture http://www.contournextstore.com/en/microlet2-colored-lancets-100s |
| Injekt 20ml luer siringe | B Braun | 4606205V | Scale https://www.soehnle-professional.com/en/productgroup/details/103/veterinary-scale |
| Askina Mullkompressen 7,5x7,5cm - sterile | B Braun | 9031219N | Sterile gauze packets in envelopes http://www.bbraun-vetcare.es/producto/askina-mullkompressen-esteril |
| Emla lidocaine/prilocaine | AstraZeneca | not available | Local anesthetics https://www.astrazeneca.es/areas-terapeuticas/neurociencias.html |
| Introcan Certo 18G short | B Braun | 4251342 | Peripheral intravenous catheter http://www.bbraun-vetcare.es/producto/introcan- |
| Introcan Certo 20G | B Braun | 4251326 | Peripheral intravenous catheter http://www.bbraun-vetcare.es/producto/introcan- |
| Blood Pressure Transducers-MA1 72-4497 | Harvard Apparatus | 724497 | Transducer for monitoring blood pressure http://www.harvardapparatus.com/physiology/physiological-measurements/transducers/pressure-transducers/research-grade-pressure-transducers.html |
| PowerLab 2/26 | AD Instruments | ML826 | Amplifier https://www.adinstruments.com/products/powerlab |
| LabChart ver. 6 | AD Instruments | not available | Acquisition software https://www.adinstruments.com/products/labchart |
| Animal Bio Amp | AD Instruments | FE136 | Amplifier https://www.adinstruments.com/products/bio-amps#product-FE136 |
| K2EDTA 7.2mg | BD | 367861 | Blood collection tubes http://catalog.bd.com/nexus-ecat/getProductDetail?productId=367861 |
| Centrifuge | SciQuip | 2-16KL | Centrifuge http://www.sigma-centrifuges.co.uk/store/products/refrigerated-sigma-2-16k-centrifuge/ |
| Eppendorf Reference 2, 100 – 1000 μL | Eppendorf | 4920000083 | Pipette https://online-shop.eppendorf.es/ES-es/Pipeteo-44563/Pipetas-44564/Eppendorf-Reference2-PF-42806.html |
| Eppendorf Safe-Lock Tubes, 0.5 mL | Eppendorf | 30121023 | Tubes https://online-shop.eppendorf.es/ES-es/Puntas-tubos-y-placas-44512/Tubos-44515/Eppendorf-Safe-Lock-Tubes-PF-8863.html |
| NZW rabbits (16-18 weeks old) | Granja San Bernardo | not available | New Zealand White rabbits http://www.granjasanbernardo.com/en/welcome/ |
| Sucrose | Sigma | S0389-5KG | Sucrose for drinking solution http://www.sigmaaldrich.com/catalog/product/sigma/s0389?lang=es®ion=ES |
| Rabbit maintenance control diet | Ssniff | V2333-000 | Control diet http://www.ssniff.com/ |
| Rabbit high-fat diet | Ssniff | S9052-E020 | High-fat diet http://www.ssniff.com/ |
| Rabbit rack and drinker | Sodispan | not available | Rack for rabbits https://www.sodispan.com/jaulas-y-racks/racks-conejo-y-cobaya/ |
| Rabbit restrainer | Zoonlab | 3045601 | http://www.zoonlab.de/en/index.html |
Odniesienia
- Cornier, M. A., Dabelea, D., Hernandez, T. L., Lindstrom, R. C., Steig, A. J., Stob, N. R., et al. The metabolic syndrome. Endocr rev. 29 (7), 777-822 (2008).
- . IDF Consensus Worldwide Definition of the Metabolic Syndrome Available from: https://www.idf.org/e-library/consensus-statements.html (2006)
- Alberti, K. G., Eckel, R. H., Grundy, S. M., Zimmet, P. Z., Cleeman, J. I., Donato, K. A., et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 120 (16), 1640-1645 (2009).
- Grundy, S. M. Pre-diabetes, metabolic syndrome, and cardiovascular risk. JACC. 59 (7), 635-643 (2012).
- Verkest, K. R. Is the metabolic syndrome a useful clinical concept in dogs? A review of the evidence. Vet J. 199 (1), 24-30 (2014).
- Zhang, X., Lerman, L. O. Investigating the Metabolic Syndrome: Contributions of Swine Models. Toxicol Pathol. 44 (3), 358-366 (2016).
- Wong, S. K., Chin, K. Y., Suhaimi, F. H., Fairus, A., Ima-Nirwana, S. Animal models of metabolic syndrome: a review. Nutr Metab (Lond). 13, 65 (2016).
- Carroll, J. F., Dwyer, T. M., Grady, A. W., Reinhart, G. A., Montani, J. P., Cockrell, K., et al. Hypertension, cardiac hypertrophy, and neurohumoral activity in a new animal model of obesity. Am J Physiol. 271 (1 Pt 2), H373-H378 (1996).
- Grooth, G. J., Klerkx, A. H., Stroes, E. S., Stalenhoef, A. F., Kastelein, J. J., Kuivenhoven, J. A. A review of CETP and its relation to atherosclerosis. J Lipid Res. 45 (11), 1967-1974 (2004).
- Zarzoso, M., Mironov, S., Guerrero-Serna, G., Willis, B. C., Pandit, S. V. Ventricular remodelling in rabbits with sustained high-fat diet. Acta Physiol (Oxf). 211 (1), 36-47 (2014).
- Filippi, S., Vignozzi, L., Morelli, A., Chavalmane, A. K., Sarchielli, E., Fibbi, B., Saad, F., Sandner, P., Ruggiano, P., Vannelli, G. B., Mannucci, E., Maggi, M. Testosterone partially ameliorates metabolic profile and erectile responsiveness to PDE5 inhibitors in an animal model of male metabolic syndrome. J Sex Med. 6 (12), 3274-3288 (2009).
- Waqar, A. B., Koike, T., Yu, Y., Inoue, T., Aoki, T., Liu, E., et al. High-fat diet without excess calories induces metabolic disorders and enhances atherosclerosis in rabbits. Atherosclerosis. 213 (1), 148-155 (2010).
- Fan, J., Watanabe, T. Cholesterol-fed and transgenic rabbit models for the study of atherosclerosis. J Atheroscler Thromb. 7 (1), 26-32 (2000).
- Yin, W., Yuan, Z., Wang, Z., Yang, B., Yang, Y. A diet high in saturated fat and sucrose alters glucoregulation and induces aortic fatty streaks in New Zealand White rabbits. Int J Exp Diabetes Res. 3 (3), 179-184 (2002).
- Zhao, S., Chu, Y., Zhang, C., Lin, Y., Xu, K., Yang, P., et al. Diet-induced central obesity and insulin resistance in rabbits. J Anim Physiol Anim Nutr (Berl). 92 (1), 105-111 (2008).
- Helfenstein, T., Fonseca, F. A., Ihara, S. S., Bottos, J. M., Moreira, F. T., Pott, H., et al. Impaired glucose tolerance plus hyperlipidaemia induced by diet promotes retina microaneurysms in New Zealand rabbits. Int J Exp Pathol. 92 (1), 40-49 (2011).
- Ning, B., Wang, X., Yu, Y., Waqar, A. B., Yu, Q., Koike, T., et al. High-fructose and high-fat diet-induced insulin resistance enhances atherosclerosis in Watanabe heritable hyperlipidemic rabbits. Nutr Metab (Lond). 12, 30 (2015).
- Liu, Y., Li, B., Li, M., Yu, Y., Wang, Z., Chen, S. Improvement of cardiac dysfunction by bilateral surgical renal denervation in animals with diabetes induced by high fructose and high fat diet. Diabetes Res Clin Pract. 115, 140-149 (2016).
- Arias-Mutis, O. J., Marrachelli, V. G., Ruiz-Saurí, A., Alberola, A., Morales, J. M., Such-Miquel, L., Monleon, D., Chorro, F. J., Such, L., Zarzoso, M. Development and characterization of an experimental model of diet-induced metabolic syndrome in rabbit. PLoS One. 12 (5), e0178315 (2017).
- Nelson, R. W., Himsel, C. A., Feldman, E. C., Bottoms, G. D. Glucose tolerance and insulin response in normal-weight and obese cats. Am J Vet Res. 51 (9), 1357-1362 (1990).
- Staup, M., Aoyagi, G., Bayless, T., Wang, Y., Chng, K. Characterization of Metabolic Status in Nonhuman Primates with the Intravenous Glucose Tolerance Test. J Vis Exp. (117), e52895 (2016).
- Hall, J. E., do Carmo, J. M., da Silva, A. A., Wang, Z., Hall, M. E. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 116 (6), 991-1006 (2015).
- Linz, D., Hohl, M., Mahfoud, F., Reil, J. C., Linz, W., Hübschle, T., Juretschke, H. P., Neumann-Häflin, C., Rütten, H., Böhm, M. Cardiac remodeling and myocardial dysfunction in obese spontaneously hypertensive rats. J Transl Med. 10 (10), 187 (2012).
- Sasser, T. A., Chapman, S. E., Li, S., Hudson, C., Orton, S. P., Diener, J. M., Gammon, S. T., Correcher, C., Leevy, W. M. Segmentation and measurement of fat volumes in murine obesity models using X-ray computed tomography. J Vis Exp. (62), e3680 (2012).
- Kawai, T., Ito, T., Ohwada, K., Mera, Y., Matsushita, M., Tomoike, H. Hereditary postprandial hypertriglyceridemic rabbit exhibits insulin resistance and central obesity: a novel model of metabolic syndrome. Arterioscler Thromb Vasc Biol. 26 (12), 2752-2757 (2006).
- Shiomi, M., Kobayashi, T., Kuniyoshi, N., Yamada, S., Ito, T. Myocardial infarction-prone Watanabe heritable hyperlipidemic rabbits with mesenteric fat accumulation are a novel animal model for metabolic syndrome. Pathobiology. 79 (6), 329-338 (2012).
- Hildrum, B., Mykletun, A., Hole, T., Midthjell, K., Dahl, A. A. Age-specific prevalence of the metabolic syndrome defined by the International Diabetes Federation and the National Cholesterol Education Program: The Norwegian HUNT 2 study. BMC Public Health. 7, 220 (2007).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone