Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
One-Step Approach to Fabricating Polydimethylsiloxane Microfluidic Channels of Different Geometric Sections by Sequential Wet Etching Processes
W tym Artykule
Podsumowanie
Several methods are available for the fabrication of channels of non-rectangular sections embedded in polydimethylsiloxane microfluidic devices. Most of them involve multistep manufacturing and extensive alignment. In this paper, a one-step approach is reported for fabricating microfluidic channels of different geometric cross sections by polydimethylsiloxane sequential wet etching.
Streszczenie
Polydimethylsiloxane (PDMS) materials are substantially exploited to fabricate microfluidic devices by using soft lithography replica molding techniques. Customized channel layout designs are necessary for specific functions and integrated performance of microfluidic devices in numerous biomedical and chemical applications (e.g., cell culture, biosensing, chemical synthesis, and liquid handling). Owing to the nature of molding approaches using silicon wafers with photoresist layers patterned by photolithography as master molds, the microfluidic channels commonly have regular cross sections of rectangular shapes with identical heights. Typically, channels with multiple heights or different geometric sections are designed to possess particular functions and to perform in various microfluidic applications (e.g., hydrophoresis is used for sorting particles and in continuous flows for separating blood cells6,7,8,9). Therefore, a great deal of effort has been made in constructing channels with various sections through multiple-step approaches like photolithography using several photoresist layers and assembly of different PDMS thin sheets. Nevertheless, such multiple-step approaches usually involve tedious procedures and extensive instrumentation. Furthermore, the fabricated devices may not perform consistently and the resulted experimental data may be unpredictable. Here, a one-step approach is developed for the straightforward fabrication of microfluidic channels with different geometric cross sections through PDMS sequential wet etching processes, that introduces etchant into channels of planned single-layer layouts embedded in PDMS materials. Compared to the existing methods for manufacturing PDMS microfluidic channels with different geometries, the developed one-step approach can significantly simplify the process to fabricate channels with non-rectangular sections or various heights. Consequently, the technique is a way of constructing complex microfluidic channels, which provides a fabrication solution for the advancement of innovative microfluidic systems.
Wprowadzenie
Microfluidic techniques have drawn attention over the past decades because of their intrinsic advantages for a variety of biomedical and chemical research and applications. Several material usage options for constructing microfluidic chips are available nowadays, such as polymers, ceramics, and silicon materials. To the best of our knowledge, among the microfluidic materials, PDMS is the most common one due to its appropriate material properties for various microfluidics research and applications, including its optical and biological compatibilities with particles, fluids, and extremely small living organisms1,2,3,4,5. Furthermore, the surface chemical and structure mechanical properties of PDMS materials can be adjusted to facilitate microelectromechanical and mechanobiological studies by applying such polymer-based microfluidic devices10,11,12. Concerning the manufacturing of microfluidic devices with designed channel patterns, soft lithography replica molding methods are usually applied to create the microfluidic channels by utilizing their corresponding master molds which are composed of photolithography-patterned photoresist layers and silicon wafer substrates12. Owing to the nature of molding approaches using silicon wafers with patterned photoresist layers, the microfluidic channels commonly have regular cross sections of rectangular shapes with identical heights.
Recently, researchers have made significant progress in biomedical studies which deal with, for instance, sorting particles and cells using hydrophoresis, separating blood plasma, and enriching white blood cells by applying microfluidic chips with channels of different heights or geometric sections6,7,8,9. Such sorting and separating functions of microfluidics for biomedical applications are realized by customizing channels with different geometric sections. Several studies have been devoted to the manufacture of microfluidic channels with cross sections of different geometry features by fabricating master molds with specific surface patterns of various heights or non-rectangular cross sections. These studies on mold fabrication include such techniques as multi-step photolithography, photoresist reflow, and grey-scale lithography13,14,15. Inevitably, the existing techniques involve finely crafted photomasks or a precise alignment in multi-step manufacturing processes, which may substantially enhance the complexity levels of the corresponding fabrication of microfluidic channels. So far, several attempts have been made on single-step manufacturing processes for microfluidic channels of various sections, but the respective techniques are highly restricted to specific cross-sectional shapes of channels16.
Over the past two decades, in addition to the molding approaches for fabricating PDMS microfluidic channels with various sections, etching techniques for patterning PDMS channels with geometric features have become the fabrication of choice in a variety of microfluidic applications. For instance, PDMS wet etching is exploited along with multi-layer PDMS bonding for constructing a pneumatic actuated cell culture device of microfluidics with reconstituted organ-level lung functions17. The PDMS wet etching technique is employed together with PDMS casting on cylindrical microwells machined by computer-aided control systems for fabricating 3D PDMS microneedle arrays18. PDMS dry etching is used to make PDMS microstructures as parts of micro-electromechanical actuators19,20. Porous PDMS membranes with designed pore layouts are also fabricated through dry etching processes21. Both the wet and the dry etching techniques can be integrated into patterning PDMS films with designated geometric shapes22.
However, the etching techniques for forming PDMS channel structures with complex section shapes have not been commonly applied because of their intrinsic limitations on microfluidic fabrication. First, while the techniques of PDMS wet etching utilizing laminar flows of chemicals for creating microfluidic channels of various sections have been established, the subsequent channel section formation is still restricted because of the basic characteristics of isotropic chemical etching processes23. Furthermore, even though there seems to be reasonable space for controlling the channel section geometries in a microfluidics fabrication using the PDMS dry etching techniques20, the required etching time is usually too long (in terms of hours) to be practical for manufacturing microfluidic chips. In addition, the etching selectivity between PDMS materials and the corresponding masking photoresist layers might be low in general, and the resulted etched depths for the channels are, thus, not acceptable20.
In this paper, we develop a one-step approach to fabricate microfluidic channels of different geometric cross sections by PDMS sequential wet etching processes (hereafter referred to as SWEP). The SWEP begin with a PDMS microfluidic device with single-layer channels. With assorted layout designs of the channels, fabricating microfluidic channels with different geometric sections of various kinds can be achieved through sequential etching processes. The sequential etching only needs an etchant to be introduced into specific channels of the planned single-layer layouts embedded in PDMS materials. Compared to conventional PDMS fabrication processes, the SWEP just require one further step to fabricate microfluidic channels of non-rectangular sections or various heights. The proposed SWEP provide a straightforward and simple way of fabricating microfluidic channels with various sections along the flow direction, which can significantly simplify the processes in the aforementioned methods.
Protokół
1. Fabrication of Microfluidic Devices with Single-Layer Channel Layouts
NOTE: In this paper, the soft lithography method3 is adopted for fabricating microfluidic devices made of PDMS materials, to demonstrate how to manufacture channels with various sections.
- Creation of master molds for a PDMS layer with designed topology features
- Design channel layouts on a PDMS layer for a single etching process or etching in sequence.
- Sketch the inverted topology features of the designed PDMS layer using a computer-aided drawing program.
- Deliver the sketch file to a photolithography facility to obtain a patterned photomask with the high-precision inverted topology features of the channel layouts printed on a transparency24.
- Use isopropyl alcohol (2-Propanol (IPA), ≥ 99.9 %), acetone (Propan-2-one, ≥ 99.5 %), and buffered oxide etch (BOE, NH4F:HF (v/v) = 6:1) on the surfaces of a 4-inch silicon wafer to remove any dust or residuals and avoid contaminations.
- Use around 500 mL of deionized water to wash the silicon wafer for a final polishing, and then apply nitrogen gas to dry the rinsed wafer.
- Place a negative tone photoresist of about 20 g on the wafer. Then spin coat the wafer at 500 rpm for 15 s and 2,000 rpm for 30 s to produce a photoresist layer of around 75 µm in thickness.
NOTE: Different photoresist thicknesses can be achieved using negative tone photoresists with different product numbers and with different spin coating, baking, and development conditions, according to the user manuals25,26. - Soft bake the wafer by heating it on a hotplate at 65 °C for 3 min and then at 95 °C for 9 min.
- Put the wafer into a photomask aligner machine along with the patterned transparency from step 1.1.3 as a mask.
- In the aligner machine, apply ultraviolet (UV) light at 300 mJ/cm2 to expose the wafer covered by the transparency.
- After exposure to the UV light, place the wafer on a hotplate at 65 °C for 2 min and then at 95 °C for 7 min as the post-exposure bake (PEB).
- Following the PEB, strongly agitate the wafer immersed in a negative tone photoresist developer, or place the immersed wafer in an ultrasonic bath (37 kHz, effective power of 180 W) for 7 min.
- Clean the entire wafer again with isopropyl alcohol to eliminate any developer remaining on the wafer surface.
- To prevent undesired bonding, silanize the surface of the wafer by putting the wafer together with 100 µL of 97% silane (1H,1H,2H,2H-perfluorooctyl-trichlorosilane) in a 6 cm Petri dish in a desiccator.
- Connect the desiccator to a vacuum pump and set the vacuum pressure at 760 mmHg.
- Next, turn the pump on for 15 min. Switch it off, and then leave the wafer to rest in a vacuum in the desiccator for 30 min.
CAUTION: The evaporated silane is extremely harmful to humans; thus, the whole wafer surface passivation must be carried out in a fume hood. - Fetch the silanized wafer, which was undergoing surface passivation. Fix the wafer in a 15 cm Petri dish for further use.
NOTE: The patterned wafer is ready to be used as a mold to replicate the designed channel layouts inversely by PDMS materials.
- Fabrication of PDMS channel layouts by replicating the inverted topology on the molds
- Put the base PDMS (monomer) along with the corresponding catalyst (curing agent) at a volume ratio of 10:1 into a clean and single-use plastic cup.
- Mix the PDMS prepolymer mixture (from step 1.2.1) homogeneously by using a power stirrer.
- Put the cup in the desiccator connected to the vacuum pump for 60 min to remove any trapped bubbles in the PDMS mixture.
- Pour 20 g (for Section 2) or 8 g (for Section 3) of the PDMS prepolymer mixture on top of the master mold (made in step 1.1) with the inverted topology features of the designed channel layouts, and then eliminate any possible bubbles embedded in the PDMS materials by using the desiccator (for 60 min).
- Put the mold carrying the PDMS mixture in an oven at 60 °C for 4 h to cure the silicone-based liquid prepolymer materials.
- After cooling the wafer together with the PDMS to room temperature for approximately 20 min, detach the cured PDMS from the mold with a scalpel and tweezers.
- Tailor the detached PDMS layer to an area (approximately 6 x 6 cm2 for Section 2 or 2 x 7.5 cm2 for Section 3) covering the entire channel layouts using a scalpel.
- Create channel access ports (inlets and outlets) by using a biopsy punch of 1.5 mm in diameter.
NOTE: The numbers and the positions of the inlets and outlets are designed based on the etching processes for fabricating specific microfluidic channels. - Pour 30 g of the PDMS prepolymer mixture into a Petri dish, and then eliminate any possible bubbles embedded in the PDMS materials by using the desiccator (for 60 min).
- Put the Petri dish carrying the PDMS mixture in an oven at 60 °C for more than 4 h to cure the liquid prepolymer materials.
- After cooling the Petri dish together with the PDMS to room temperature for approximately 20 min, detach the cured PDMS from the dish with a scalpel and tweezers.
- Using a scalpel, tailor the detached PDMS layer without any features to dimensions equal to those of the aforementioned PDMS layer (approximately 6 x 6 cm2 for Section 2 or 2 x 7.5 cm2 for Section 3).
- Activate the surfaces of both PDMS layers (made in steps 1.2.7 and 1.2.12) with the designed channel layouts and without any features by exposing the top PDMS materials to oxygen plasma in a surface treatment machine at 90 W for 40 s.
- Bond the 2 PDMS layers by making contact between their treated surfaces right after the oxygen plasma surface activation. Then, leave the bonded PDMS layers in an oven at 60 °C for more than 30 min.
NOTE: There is no upper time limit for leaving the bonded PDMS layers in the oven. - After the 2 bonded PDMS layers have cooled, trim the excess PDMS materials away from the fabricated device for a later experimental set-up.
2. The One-Step Approach to Fabricating PDMS Microfluidic Channels of Different Sections
NOTE: To characterize the PDMS wet etching rate, a microfluidic device with a single-layer and straight channel of rectangular shapes is suggested to be exploited for identifying specific etching rates corresponding to certain experimental settings.
- Experimental characterization of PDMS wet etching
- Prepare an etchant solution by mixing tetra-n-butylammonium fluoride (TBAF, a 1 M solution in tetrahydrofuran (THF)) with 1-Methyl-2-pyrrolidinone (NMP) at a rate of v:v = 1:10.
NOTE: NMP is capable of efficiently dissolving chemical residuals induced by the etchants. In general, PDMS materials are swollen marginally by the NMP, and the PDMS microfluidic devices are still able to preserve their shapes, volumes, and seal conditions. - Draw the mixed TBAF/NMP etchants into a 10 mL syringe connected to a stainless blunt needle (16 G).
- Set up a syringe pump as a controller of the pressure-driven fluids in the channels.
- Connect the blunt needles of the syringes filled with the etchant solution to the channel port of the abovementioned simple device and guide the respective port from the outlet tubing to a waste container as shown in Figure 1.
- Run the syringe pump carrying the syringes containing the mixed TBAF/NMP etchant solution at a 150 µL/min flow rate for characterizing the PDMS wet etching.
- Use bright-field microscopic views and make sure that the etched channel along the flow direction has a uniform width, to consequently confirm that the volume mixing ratio of the etchants and the etchant flow rate are adequate.
- Capture the time-series images of the channel cross section under an inverted microscope with a 4X magnification during the PDMS etching process.
- Analyze the stored images by applying the basic measurement function in a 2D analysis of the imaging processing program to collect a time sequence of numbers for the channel width during the wet etching process of PDMS materials.
- Evaluate the time-series etching rates through the equation shown in Figure 2, which is dividing 50% of the channel width change (ΔW / 2) by the duration of the PDMS etching (t).
- Perform a linear regression of the collected data points to estimate an overall etching rate of the mixed TBAF/NMP etchants with the specific volume mixing ratio of 1:10 for the PDMS materials as shown in Figure 2.
- Prepare an etchant solution by mixing tetra-n-butylammonium fluoride (TBAF, a 1 M solution in tetrahydrofuran (THF)) with 1-Methyl-2-pyrrolidinone (NMP) at a rate of v:v = 1:10.
- PDMS sequential wet etching for fabricating microfluidic channels of different geometric sections
- Design an arrangement of etchant inlets for the single-layer PDMS channel layout serving the corresponding etching processes in sequence, so that a specific channel type of different cross-sectional shapes as shown in Figure 3 can be fabricated.
- Follow the procedures described in steps 2.1.1 - 2.1.7 for the PDMS wet etching approach.
NOTE: The flow rate is set as 50 μL/min. - While the TBAF/NMP etchants are flowing, inspect the etched channels under the microscope to see if there exist significant problems such as a noticeable amount of bubbles, a remaining of several chemical residuals induced by the etchants, a leakage of the etchants, or a flow of etchants on an inclined plane.
- Observe the microfluidic channel wall thickness variation by inverted microscopy, and time the wet etching process to ensure the proper channel geometries are achieved.
3. The Design of a Microfluidic Mixer
NOTE: A design of the microfluidic mixer which can efficiently mix 2 dissimilar fluids is demonstrated here to show an advantageous application of microfluidic channels with different sections.
- Fabrication of a microfluidic mixer with different channel sections
- Make a PDMS device with a single-layer microfluidic channel of the design shown in Figure 4 by the soft lithography replica molding technique (section 2).
- In the single-layer microfluidic channel layout, introduce the TBAF/NMP etchant solution prepared by following the procedures described in step 2.1.1 from the port marked as "outlet" at a 20 µL/min flow rate in Figure 4.
- Observe the microfluidic channel wall thickness variation under the microscope, and time the wet etching process to ensure the proper channel geometries as represented in Figure 5 are achieved.
- Experimental characterization of the microfluidic mixer
- After the microfluidic channel with sections of different shapes in an alternate pattern is realized, pump 2 dissimilar fluids including a solution of fluorescein sodium salt having a 50 µg/mL concentration and distilled water into 2 separate channels at a 20 µL/min flow rate.
- Take fluorescence microscope images of the channel in top view at the positions marked as A, B, C, and D under an inverted microscope (4X magnification) for the 2 mixers with uniform (before etching) and different geometric sections (after 2 h of SWEP), respectively (Figure 6).
NOTE: The fluorescence microscope images are taken while the stable flows occur, at the time point of 5 min, counted from the beginning moments of mixing through the mixer channels. - Analyze the captured fluorescent images by using an imaging processing program to estimate the corresponding mixing efficiency numbers which are defined by the mixing residual (MR, 0.5 = unmixed, 0 = fully mixed) in the following equation27,28:
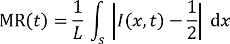
Here,
t is the etching time,
L is the channel width at a certain position of interest,
S is a line segment across the channel at the position, and
I is the fluorescence intensity distribution over S at t. - Plot the fluorescence intensity distribution over S across the channel at the positions marked as A, B, C, and D for the 2 mixers with uniform (before etching) and different geometric sections (after 2 h of SWEP), respectively. Estimate the corresponding MR as depicted in Figure 6.
Wyniki
Recently, a large number of studies have been made on the fabrication of microfluidic devices with channels of different sections by lithography replica molding13,14,15 and PDMS etching techniques17,18,19,20,21,
Dyskusje
Over the past decades, microfluidics has offered promising means by which experimental platforms for chemical and biomedical research can be constructed systematically1,2,3,4,5. The platforms have also presented their capabilities of investigating several cellular functions in vivo under physiological microenvironment conditions via in vitro ...
Ujawnienia
The authors have nothing to declare.
Podziękowania
The authors gratefully acknowledge the support provided by the National Health Research Institutes (NHRI) in Taiwan under the Innovative Research Grant (IRG) (EX106-10523EI), the Taiwan Ministry of Science and Technology (MOST 104-2218-E-032-004, 104-2221-E-001-015-MY3, 105-2221-E-001-002-MY2, 105-2221-E-032-006, 106-2221-E-032-018-MY2), and the Academia Sinica Career Development Award. The authors would like to thank Heng-Hua Hsu for proofreading the manuscript.
Materiały
| Name | Company | Catalog Number | Comments |
| 1-Methyl-2-Pyrrolidinone | Tedia, Fairfield, OH | ME-1962 | NMP |
| 10 ml Syringe | Becton-Dickinson, Franklin Lakes, NJ | 302151 | |
| 150 mm Petri dish | Dogger Science | DP-43151 | |
| 1H,1H,2H,2H- Perfluorooctyltrichlorosilane | Alfa Aesar, Ward Hill, MA | L16606 | 97 % silane |
| 4'' Silicon Dummy Wafer | Wollemi Technical, Taoyuan, Taiwan | - | |
| Acetone | ECHO Chemical, Miaoli, Taiwan | AH3102-000000-72EC | |
| AG Double Expose Mask Aligner | M&R Nano Technology, Taoyuan, Taiwan | AG500-4D-D-V-S-H | |
| Biopsy Punch | Miltex, Plainsboro, NJ | 33-31 | |
| Blunt Needle | Jensen Global, Santa Barbara, CA | Gauge 16 | |
| Buffered Oxide Etch | ECHO Chemical, Miaoli, Taiwan | PH3101-000000-72EC | |
| Desicattor | A-VAC Industries, Anaheim, CA | 35.10001.01 | |
| Fluorescein Sodium Salt Water | Sigma-Aldrich Co., St Louis, MO | F6300 | |
| ImageJ | National Institutes of Health, Bethesda, MD | Ver. 1.51 | Imaging Processing Program |
| Inverted Fluorescence Microscope | Leica Microsystems, Wetzlar, Germany | DMI 6000 B | |
| Isopropyl Alcohol (IPA) | ECHO Chemical, Miaoli, Taiwan | CMOS112-00000-72EC | |
| Leica Application Suite | Leica Microsystems GmbH | LAS X | |
| MATLAB | MathWorks, Natick, MA | R2015b | Programming for MR evaluation |
| Mechanical Convention Oven | ThermoFisher Scientific,Waltham, MA | Lindberg Blue M MO1450C | |
| Plasma Tretment System | Nordson MARCH, Concord CA | PX-250 | Oxygen plasma surface treatment |
| Polydimehtylsiloxane (PDMS) | Dow Corning, Midland, MI | SYLGARD 184 | |
| Polyethylene Tubing | Becton-Dickinson and Company, Sparks, MD | 427446 | PE 205, 10' |
| Spin Coater | ELS Technology, Hsinchu, Taiwan | ELS 306MA | |
| Negative Tone Photoresist | MicroChem, Westborough, MA | SU-8 2050 | |
| Negative Tone Photoresist Developer | MicroChem, Westborough, MA | Y020100 | SU-8 Developer |
| Surgical Blade | Feather, Osaka, Japan | 5005093 | PDMS cutting |
| Syringe Pump | Chemyx, Houston, TX | Fusion 400 | |
| Tetra-n-butylammonium Fluoride (TBAF) | Alfa Aesar, Ward Hill, MA | A10588 |
Odniesienia
- Tung, Y. -. C., et al. Optofluidic Detection for Cellular Phenotyping. Lab on a Chip. 12, 3552-3565 (2012).
- Lu, Y., Yang, L., Wei, W., Shi, Q. Microchip-based Single-cell Functional Proteomics for Biomedical Applications. Lab on a Chip. 17, 1250-1263 (2017).
- Jensen, K. F., Reizman, B. J., Newman, S. G. Tools for Chemical Synthesis in Microsystems. Lab on a Chip. 14, 3206-3212 (2014).
- Chang, C. -. W., et al. A Polydimethylsiloxane-polycarbonate Hybrid Microfluidic Device Capable of Generating Perpendicular Chemical and Oxygen Gradients for Cell Culture Studies. Lab on a Chip. 14, 3762-3772 (2014).
- Mosadegh, B., et al. Integrated Elastomeric Components for Autonomous Regulation of Sequential and Oscillatory Flow Switching in Microfluidic Devices. Nature Physics. 6, 433-437 (2010).
- Choi, S., Park, J. -. K. Tuneable Hydrophoretic Separation Using Elastic Deformation of Poly(Dimethylsiloxane). Lab on a Chip. 9, 1962-1965 (2009).
- Choi, S., Song, S., Choi, C., Park, J. -. K. Microfluidic Self-Sorting of Mammalian Cells to Achieve Cell Cycle Synchrony by Hydrophoresis. Analytical Chemistry. 81, 1964-1968 (2009).
- VanDelinder, V., Groisman, A. Separation of Plasma from Whole Human Blood in a Continuous Cross-Flow in a Molded Microfluidic Device. Analytical Chemistry. 78, 3765-3771 (2006).
- VanDelinder, V., Groisman, A. Perfusion in Microfluidic Cross-Flow: Separation of White Blood Cells from Whole Blood and Exchange of Medium in a Continuous Flow. Analytical Chemistry. 79, 2023-2030 (2007).
- Duffy, D. C., McDonald, J. C., Schueller, O. J., Whitesides, G. M. Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane). Analytical Chemistry. 70 (23), 4974-4984 (1998).
- Xia, Y., Whitesides, G. M. Soft Lithography. Annual Review of Material Science. 28, 153-184 (1998).
- Mello, A. Plastic Fantastic?. Lab on a Chip. 2, 31N-36N (2002).
- Choi, S., Park, J. -. K. Two-step Photolithography to Fabricate Multilevel Microchannels. Biomicrofluidics. 4, 046503 (2010).
- Zhong, K., Gao, Y., Li, F., Zhang, Z., Luo, N. Fabrication of PDMS Microlens Array by Digital Maskless Grayscale Lithography and Replica Molding Technique. Optik. 125, 2413-2416 (2013).
- Brower, K., White, A. K., Fordyce, P. M. Multi-step Variable Height Photolithography for Valved Multilayer Microfluidic Devices. Journal of Visualized Experiments. (119), e55276 (2017).
- Lai, D., et al. Simple Multi-level Microchannel Fabrication by Pseudo-grayscale Backside Diffused Light Lithography. RSC Advances. 3, 19467-19473 (2013).
- Huh, D., et al. Reconstituting Organ-Level Lung Functions on a Chip. Science. 328, 1662-1668 (2010).
- Deng, Y. -. L., Juang, Y. -. J. Polydimethyl Siloxane Wet Etching for Three-Dimensional Fabrication of Microneedle Array and High-Aspect-Ratio Micropillars. Biomicrofluidics. 8, 026502 (2014).
- Tung, Y. -. C., Kurabayashi, K. Nanoimprinted Strain-controlled Elastomeric Gratings for Optical Wavelength Tuning. Applied Physics Letters. 86, 161113 (2005).
- Tung, Y. -. C., Kurabayashi, K. A Single-Layer PDMS-On-Silicon Hybrid Microactuator with Multi-Axis Out-Of-Plane Motion Capabilities-Part II: Fabrication and Characterization. Journal of Microelectromechanical Systems. 14, 558-566 (2005).
- Chen, W., Lam, R. H. W., Fu, J. Photolithographic Surface Micromachining of Polydimethylsiloxane (PDMS). Lab on a Chip. 12, 391-395 (2012).
- Balakrisnan, B., Patil, S., Smela, E. Patterning PDMS Using a Combination of Wet and Dry Etching. Journal of Micromechanics and Microengineering. 19, 047002 (2009).
- Takayama, S., et al. Topographical Micropatterning of Poly(dimethylsiloxane) Using Laminar Flows of Liquids in Capillaries. Advanced Materials. 13, 570-574 (2001).
- Friend, J., Yeo, L. Fabrication of Microfluidic Devices Using Polydimethylsiloxane. Biomicrofluidics. 4, 026502 (2010).
- . NANO SU-8 2000 Negative Tone Photoresist formulations 2002-2025 Available from: https://www.seas.upenn.edu/~nanosop/documents/SU8_2002-2025.pdf (2000)
- . NANO SU-8 2000 Negative Tone Photoresist formulations 2035-2100 Available from: https://www.seas.upenn.edu/~nanosop/documents/SU8_2035-2100.pdf (2000)
- Hardt, S., Schönfeld, F. Laminar Mixing in Different Interdigital Micromixers: II. Numerical Simulations. AIChE Journal. 49, 578-584 (2003).
- Hessel, V., Löwe, H., Schönfeld, F. Micromixers-A Review on Passive and Active Mixing Principles. Chemical Engineering Science. 60, 2479-2501 (2005).
- Damiati, S., Kompella, U., Damiati, S., Kodzius, R. Microfluidic Devices for Drug Delivery Systems and Drug Screening. Genes. 9, 103 (2018).
- Wang, C. -. K., et al. Single Step Sequential Polydimethylsiloxane Wet Etching to Fabricate a Microfluidic Channel with Various Cross-Sectional Geometries. Journal of Micromechanics and Microengineering. 27, 115003 (2017).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone