Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
A New Portable In Vitro Exposure Cassette for Aerosol Sampling
W tym Artykule
Podsumowanie
Here, we present a protocol to perform portable cellular aerosol exposures and measure cellular response. The method uses cells, grown at the air-liquid interface, mimicking in vivo physiology. Cellular response to copper nanoparticle aerosols was observed as oxidative stress through reactive oxygen species generation and cytotoxicity as lactate dehydrogenase release.
Streszczenie
This protocol introduces a new in vitro exposure system, capable of being worn, including its characterization and performance. Air-liquid interface (ALI) in vitro exposure systems are often large and bulky, making transport to the field and operation at the source of emission or within the breathing zone difficult. Through miniaturization of these systems, the lab can be brought to the field, expediting processing time and providing a more appropriate exposure method that does not alter the aerosol prior to contacting the cells. The Portable In vitro Exposure Cassette (PIVEC) adapts a 37 mm filter cassette to allow for in vitro toxicity testing outside of a traditional laboratory setting. The PIVEC was characterized using three sizes of copper nanoparticles to determine deposition efficiency based on gravimetric and particle number concentration analysis. Initial cytotoxicity experiments were performed with exposed lung cells to determine the ability of the system to deposit particles while maintaining cell viability. The PIVEC provides a similar or increased deposition efficiency when comparing to available perpendicular flow in vitro exposure devices. Despite the lower sample throughput, the small size gives some advantages to the current in vitro ALI exposure systems. These include the ability to be worn for personal monitoring, mobility from the laboratory to the source of emission, and the option to set-up multiple systems for spatial resolution while maintaining a lower user cost. The PIVEC is a system capable of collecting aerosols in the field and within the breathing zone onto an air-interfaced, in vitro model.
Wprowadzenie
Personal sampling using in vitro techniques could provide comprehensive information regarding the biological effects of aerosols in the workplace.1 Exposures to contaminants in the air include exposures to the chemical itself, to the collected air samples, under submerged conditions where the gas is introduced to the cell suspension, intermittent exposures using a device such as a rocker, or direct exposures at the air-liquid interface (ALI).2 Many of these techniques are performed with cells grown in suspension or the collection of samples prior to the exposure, each of which can affect the toxicological study due to potential changes in the aerosol.3 To avoid these changes, the laboratory can be brought to the field using several in vitro ALI culture exposure systems that are used in literature,4,5,6,7,8,9,10,11,12,13 however, few are commercially available.8,9,12 These systems are often bulky, especially when including instruments to regulate the temperature and humidity of the cellular environment and the flow rate of the sample aerosol. By using the PIVEC, aerosol exposures can be performed outside of a traditional lab setting or within the breathing zone while mimicking inhalation conditions.
The determination of aerosol deposition in vitro is important to the investigation of health effects due to inhalation. The breathing zone, the area within 30 cm from the mouth and nose,14 is crucial for understanding the exposure to nanoparticles and for linking to the biological effects in the lungs.2 Often, the deposition on cells is defined as a deposition efficiency, the particles deposited onto and taken up by the cells divided by the particles administered to the system6,15 or on a mass basis of the same amounts.4,16 The current methods for measuring aerosols in the breathing zone are filter based, capturing particles over a given sampling period and using the filters to conduct further testing.17 Personal monitoring requires a small system that comes with the tradeoff of fewer samples.
There are many approaches to determine the health effects from exposure to an aerosol. The ALI model allows for the aerosol to be administered directly to the cells through the air as in a real exposure scenario, yet it is more cost-effective and less time intensive than in vivo studies while mimicking the air-liquid barriers such as the eyes, skin, and lungs. Lung cells grown at the ALI have the ability to generate a polarized barrier layer,18,19 which produces physiological traits that resemble the in vivo lung epithelium, including mucus and surfactant production in specific bronchial or alveolar cell lines, cilia beating,19 tight junctions,19,20 and cell polarization.18 Changes such as these can affect the cellular response measured in toxicity studies.21 In addition, ALI in vitro model results are often more sensitive than cells exposed via suspension models22 and are able to model acute in vivo inhalation toxicity.23,24 Therefore, an ALI exposure system that is able to perform measurements within the breathing zone is a natural next step.
By exposing the cells to aerosol directly at the source of emission, investigation of the effects of all gases, semi-volatile compounds, and particles involved in the mixture occurs. When the mixture is collected on a filter, the gases and volatile compounds are not captured and the whole mixture cannot be investigated. In addition, reconstitution of particles into a powder or a liquid suspension can lead to the aggregation or particle-fluid interactions, such as dissolution, in liquid suspension.25,26 When aerosol particles are added to the liquid, there is a higher potential for agglomeration,25,27 formation of a protein corona,28 or interaction with compounds in the liquid, which can affect deposition and influence the biological response.29,30
Exposure at the ALI is based on three main aerosol profiles, cloud settling, parallel flow, and perpendicular flow. Cloud settling, used by the Air-Liquid Interface Cell Exposure (ALICE),4 is a batch system where particles deposit through gravitational and diffusional settling as the aerosol is treated as one unit. Parallel flow, used by the Electrostatic Aerosol in vitro Exposure System (EAVES)5 and Multiculture Exposure Chamber (MEC) II,6 allows for deposition through the addition of Brownian motion through the flow profile. Perpendicular flow, used by a microsprayer,7 Nano Aerosol Chamber for In-Vitro Toxicity (NACIVT),11 and commercial ALI systems8,9,10,12, adds the impaction of particles within the deposition region. Many of these exposure systems are large and bulky, requiring excess systems for aerosol pre-conditioning, pumps for flow, or even heating chambers for incubation of cells. This large size decreases the portability of the system. Instead of sampling directly at the source of emission, these systems often have samples brought to the lab or model aerosols generated for analysis. The complexity of the emitted aerosol can be lost in translation from the field to the lab. The PIVEC is smaller than current systems, with an external surface area of approximately 460 cm2 and weighing only 60 grams, with thermal and humidity control incorporated into the system allowing for a highly portable device. The decreased size and weight allow the system to be worn or taken to the source of exposure, permitting direct sampling.
The large size of current exposure systems also decreases the ability to perform sampling to investigate spatial gradients in concentrations. This resolution is key when determining toxicological effects of many potential environmental and occupational hazards such as vehicular exhaust particulate matter or workplace activities where aerosolization occurs. Immediately post-emission, there becomes a spatial variance in particle concentration. This grows with time as the particles disperse throughout the atmosphere and these effects can change based on the ambient conditions, such as temperature, pressure, wind, and sun. Particles can begin to age and oxidize as well once emitted31,32 and dispersal rates are affected by the topography; higher concentrations will be found in canyons and tunnels, where dispersion effects are slowed, and lower concentrations can be found where there is a large area for dispersion.33 These changes in dispersion rates can have significant effects on human health and can be seen when comparing the number of asthmatic adults living in urban versus in rural settings.34 While many exposure systems provide multiple samples at once, multiple systems are necessary with an abundance of large equipment to perform spatial resolution.
By bringing the lab to the field, the time of analysis can be decreased by using the whole cell as a sensor. Following known biological mechanisms and endpoints can aid in the determination of the aerosol composition and size. Due to slow clearance methods, including mucociliary clearance, phagocytosis, and translocation, these particles are often interacting with cells for approximately days to weeks3 generating oxidative stress, inflammation, and even cell death. These biological endpoints can be the starting points for adverse outcome pathways for cardiovascular disease or chronic obstructive pulmonary disease. In addition, Wiemenn et al. performed an array of in vitro assays to compare with literature values for short term in vivo inhalation toxicity.35 In vivo response was predicted with two of four positive results from testing cytotoxicity via lactate dehydrogenase release, oxidative stress from glutathione reduction and hydrogen peroxide formation and release, and inflammation potential from the tumor necrosis factor alpha gene. Out of ten nanosized metal oxides tested, six tested as active (titanium oxide, zinc oxide, and four different cerium oxide) using exposures in vitro with confirmation in vivo.
In order to study the effects of aerosols in an occupational setting, our lab developed the PIVEC for exposures in the field. Additionally, the PIVEC can be worn for personal sampling to monitor and investigate inhalation exposure like the 37 mm filter cassette36 or multiple systems can be used to achieve spatial resolution within a given area. In this protocol, the characterization and use of the PIVEC is discussed. After exposure, the biological effects are observed through cytotoxicity assays.
Protokół
Operators must wear personal protective equipment (e.g. lab coat, gloves, goggles) when performing steps 1, 2, 3, 5, and 6.
1. Preparation of Materials
- Prepare materials for system assembly and exposure to ensure repeatability.
- Make sure to use new or 70% ethanol cleaned ¼” inner diameter conductive tubing and ¼” outer diameter connectors for the system assembly.
- Store test materials including filters, PIVEC components, tweezers, and particle powders in a well-controlled environment, with respect to the temperature and humidity, for at least 24 h prior to the experiment.
NOTE: The temperature should be near room temperature, approximately 20 °C, with relative humidity less than 35%. This is very important to achieve repeatability between experiments. - Prepare particle counters using isopropanol to clean parts and allow for the system warm-up according to the manufacturer recommendations, including the scanning mobility particle sizer (SMPS) and optical particle sizer (OPS) for measurement.
2. Generation of Dry Aerosol
NOTE: Operators should perform aerosol generation in a fume hood.
- Assemble a system to generate dry aerosols
NOTE: The suspension of particles in gas or liquid should be appropriate for the modeled application and cell culture. The following method can be performed using a liquid-based aerosol. The design of the dry aerosol system is from Tiwari et al.37 A schematic of the dry dispersal system is shown in Figure 1.- Connect the ball valve to each end of the 4” 1/8 size threaded pipe, this will serve as the particle hopper. Connect 2” 1/8 size pipe to one valve.
- Weigh copper nanoparticles, in this study the mass concentration for each particle size was kept constant while determining the deposition efficiency. Approximately use 7.5 mg of 40 nm copper nanoparticles, 7 mg of 100 nm copper nanoparticles, and 13 mg of 800 nm copper nanoparticles per exposure. Place copper nanoparticles into the particle hopper through the open end.
NOTE: The amount of copper nanoparticles weighed will serve as the mass based administered concentration. - Place a 3” piece of ½” outer diameter (OD) tubing around the 2” pipe and place a HEPA filter inside this short tubing such that the flow direction is through the ball valve.
- Connect the vacuum generator to other ball valve using threading. Connect vacuum generator to air tank by placing a 5/16” OD tubing into the push-to-lock connection. Use ¼” OD tubing to connect the outlet of the vacuum generator to the experimental set-up by placing the tubing over the outlet of the vacuum generator.
- Use of dry aerosol system to generate dry aerosol
- Open the air tank by turning the main valve and allow the air flow to the system. Open the valve on the flow regulator on the air tank and set such that the flow through the system is equivalent to the desired settings on the vacuum pump.
- Open the ball valve closest to HEPA filter then open the ball valve closest to vacuum generator. Keep these open for approximately 3 s to allow particles to be pulled into the air stream.
- Close the ball valve closest to vacuum generator then close ball valve closest to HEPA filter. Allow air from the tank to flow for the duration of the experiment as necessary.
- Close main and regulator valves on air tank to stop the flow. Clean ball valves and vacuum generator using 70% ethanol. Autoclave metal pipes for sterilization.
3. Deposition Efficiency Measurement using PIVEC
NOTE: Operators should perform aerosol exposures in a fume hood.
- Measure the deposition by collecting the copper nanoparticle aerosol generated in step 2.2 on a pre-weighed filter. Use the deposited dose, measured using the collected mass on the filter, and the administered dose, measured using the amount of weighed copper particles, to determine the deposition efficiency.
- Keep 1.00 µm pore glass fiber filters under low humidity conditions, described in 1.1.2, for at least 24 h prior to pre-exposure measurements. Weigh an unused filter three times and record the filter weights. Place the unused filter in a cell culture insert.
- Choose appropriate cell culture insert adapter (6 well or 24 well) for PIVEC to support the cell culture insert with the filter. Place the cell culture insert adapter piece on the top of the base of PIVEC, setting into place such that the base of the adapter piece is wider than the top.
- Use tweezers to place filter loaded cell culture insert within adapter piece. Place the top piece on top of adapter piece, settling into place such that the base of the top piece is wider than the top. Wrap PIVEC with a single layer of duct tape.
- Connect 37 mm cassette pieces on top and bottom of PIVEC by pushing into place. Place ¼” barbed adapters into cassette inlet and outlet.
- Wrap the resistive heater around PIVEC such that the wires are at the base. Tape to secure.
- Wrap PIVEC with ~8 rounds of aluminum foil for insulation. Secure with tape.
- Connect 2” long piece of 1/2” outer diameter flexible tubing to the adapter on top of PIVEC. Remove porous tubing from sterile water and place within tubing on top of PIVEC.
- Place PIVEC within clamp on the ring stand and secure. Complete set-up with the vacuum pump, particle counters, and aerosol set-up.
NOTE: The number-based deposited dose can be determined only if particle counters are placed before the PIVEC and after the PIVEC on separate runs. - Expose filters using step 2.2 of protocol and desired exposure time and flow rates, in this study an exposure time of 10 min at 0.5 LPM was used. Remove PIVEC from the set-up. Take out the cell culture insert and place the exposed filter in filter holder under low humidity conditions for at least 24 h prior to measurements.
- Clean PIVEC with 70% ethanol. Sterilize with ultraviolet light for at least 30 min prior to the next experiment.
- Weigh the exposed filter three times and record the filter weights. Place the exposed filter in a labeled filter holder for storage.
4. Calculation of Deposited Dose and Deposition Efficiency
NOTE: Knowledge of the deposition is important for aerosol administration and interpretation of cellular response.
- Calculate deposition from mass-based measurements
- Calculate the deposited mass on filters as the difference between the pre-exposure average weight and post-exposure average weight. This value is the mass-based deposited dose for the experiment.
- Use administered mass, madmin, and mass-based deposited dose determined in 4.1.1, mdep, to calculate the mass-based deposition efficiency, ηm, for the experiment.
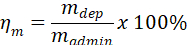
- Average values from 4.1.1 and 4.1.2 for at least 3 experiments to determine deposition and deposition efficiency for PIVEC for particle size.
- Calculate deposition from number-based measurements
- Ensure that measurements with particle counters have been performed with counters after the PIVEC and to determine the particle concentration before the PIVEC. Integrate the particle concentration over time for the particle counter then integrate over particle diameter to determine the total particles measured.
- Calculate the deposited particle number as the difference between the particles administered and the particles measured post-PIVEC. This value is the number-based deposited dose for the experiment.
- Use administered particles, nadmin, number-based deposited dose, ndep, volumetric flow rate, V, and time, t, to calculate the number-based deposition efficiency, ηn, for the experiment.
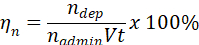
- Average values from 4.2.2 and 4.2.3 for at least 3 experiments to determine deposition and deposition efficiency for PIVEC for particle size.
5. Aerosol Exposure of Cells
NOTE: For the cell culture at the air-liquid interface the reader is referred to Blank et al.38 Operators should perform cell culture insert loading (steps 5.1.2-5.1.4) within a biosafety cabinet. Operators should perform aerosol exposures in a fume hood.
- Culture Cells at Air-Liquid Interface
- Lift A549 cells from culture flask by adding trypsin-EDTA, 3 mL for a T75 flask or 1 mL for a T25 flask and incubate for 5 min at 37 °C. Add 7 mL of complete media for a T75 flask or 4 mL of complete media for a T25 flask to flask and rinse flask wall with cell suspension to maximize the recovered cell number. Transfer the cell suspension to a sterile 15 mL conical tube then centrifuge cells at 800 x g for 3 min.
- Remove the supernatant containing trypsin-EDTA and resuspend the cell pellet in 10 mL of complete media. Remove 10 µL of cell suspension and add to the hemocytometer. Count cells using a hemocytometer to determine the concentration and the total number of cells.
- Place 0.5 mL of complete media to each well within a 24 well plate. Place unused cell culture inserts in wells. Seed cell culture inserts on the apical side at a cell density near 1 x 105 cells/cm2 for cell types that grow at a rate near doubling per day. To seed A549 cells within a 24 well insert, seed cells at a density of 1 x 105 cells/cm2 by adding 35,000 cells to the cell culture insert.
NOTE: Cells with a slower growth rate can be seeded at an increased cell density. - Add complete media to the apical side of cell culture insert to reach the final volume (for 24 well plate final volume is 0.25 mL).
- Culture for 7 days in submerged conditions, replacing media every 1-2 days. After 7 days, remove the apical media and culture for at least 1 day in ALI conditions, replacing only the basolateral media.
- Assemble PIVEC
- Allow cells to equilibrate to the air-liquid interface for at least 24 h prior to exposure.
- Choose appropriate cell culture insert adapter for PIVEC to support the cell culture insert with the filter. Place cell culture insert adapter piece on top of PIVEC base, setting into place such that the base of the adapter piece is wider than the top. Add 4 mL of cell culture media to the well of the base of the PIVEC.
- Use tweezers to place the cell culture insert within adapter piece placed in step 5.2.3. Place top piece on top of adapter piece, settling into place such that the base of the top piece is wider than the top. Carefully, wrap PIVEC with a single layer of duct tape.
- Connect 37 mm cassette pieces on top and bottom of PIVEC, by pushing into place. Place ¼” barbed adapters into cassette inlet and outlet.
- Wrap resistive heater around PIVEC such that the wires are at the base. Tape to secure.
- Wrap PIVEC with ~8 rounds of aluminum foil for insulation. Secure with tape.
- Connect short piece of 1/2” outer diameter flexible tubing to the adapter on the top of PIVEC. Remove porous tubing from sterile water and place within tubing on top of PIVEC.
- Place PIVEC within clamp on the ring stand and secure. Complete the set-up with the vacuum pump and aerosol set-up.
- Expose Cells at ALI using the PIVEC
- Use deposition efficiency determined in Step 2 to calculate the mass of particles to be aerosolized. Weigh appropriate mass and add to the aerosol system set up following step 2 within the fume hood.
- Expose cells by following step 2.2, in this study of biological endpoints the cells were exposed to approximately 3.5 mg of copper nanoparticles with a flow rate of 0.5 LPM and an exposure duration of 10 min. Control studies performed used humidified air to determine the influence of the air alone. Remove PIVEC from the set-up. Take out the cell culture insert, place in the sterile well plate and return to the CO2 incubator (37°C, 5% CO2, 90% RH).
- Aspirate media from PIVEC. If performing additional experiments, rinse bottom of PIVEC with phosphate buffered solution then repeat step 5.1 and 5.2.
- Clean PIVEC with 70% ethanol when finished. Sterilize with ultraviolet light for at least 30 min prior to the next experiment.
- Biological Assay Procedures
NOTE: Assays performed in this study were oxidative stress generation through the DCFH-DA assay and cytotoxicity through lactate dehydrogenase (LDH) release.- Dissolve 24.4 mg of DCFH-DA in 50 mL methanol to make 1 mM DCFH-DA solution. This solution can be stored at -20°C for up to 4 months. Dilute 1 mM DCFH-DA solution by mixing 0.1 mL of 1 mM DCFH-DA solution with 9.9 mL HBSS to make 10 mL of 10 µM DCFH-DA.
- Remove cell culture media and wash cell culture insert with approximately 1 mL of PBS. Add 0.5 mL of 10 µM DCFH-DA solution to each well, replacing inserts when finished. Cover plate with aluminum foil to prevent photoactivation of the dye and return to 37°C incubator for 1 h.
- Remove cells from the incubator and aspirate the DCFH-DA working solution from the wells. Add 0.5 mL HBSS to wells and replace cell culture inserts.
- Load the well plate into plate reader and measure baseline fluorescence using excitation/emission wavelengths of 485/530 nm. Remove plate from plate reader and load insert into PIVEC for exposure.
- Expose cells for desired exposure duration. Remove insert from PIVEC and return to well plate. Remove 50 µL of basolateral fluid from well plate and place in white 96 well plate. Measure the fluorescence of DCF using excitation/emission wavelengths of 485/530 nm every 30 min post-exposure for 2 h.
- Let basolateral fluid equilibrate to room temperature for 20-30 min. Add 50 µL of LDH assay solution, mixed following manufacturer protocol, to basolateral fluid from well plate and let react for 10 min. Add 25 µL of stop solution to well. Read fluorescence of resorufin product using excitation/emission wavelengths of 560/590 nm.
- Remove additional basolateral fluid and repeat step 5.4.6 at 4 h and 24 h post-exposure.
6 Statistical Methods
- Analysis of Biological Assay Data
- Report ROS production as the fluorescence intensity increase of treated cells relative to baseline measurements. Report LDH activity as the fluorescence intensity increase of treated cells relative to untreated cells.
- Perform single factor ANOVA to determine statistical differences between data sets. Where appropriate, perform student t-tests at a value of significance of 0.05. Report data as the mean ± standard deviation of at least three exposure measurements.
Wyniki
Occupational in vitro toxicology involves maintaining cellular viability while performing aerosol exposure. The PIVEC system is shown in Figure 2, including the temperature and humidity control and the worn PIVEC. The temperature was maintained using a battery-powered resistive heater and the aerosol humidified using increased natural humidification through a porous, wetted tube. In a controlled aerosol setting inside a laboratory, the PIVEC can be s...
Dyskusje
Filter cassettes provide a simple, inexpensive method of collecting aerosols in the breathing zone; however, aerosol samples extracted from filters do not represent the entire aerosol (i.e. gases, volatiles, and particulates) and consequently limit the assessment of related biological effects. Using the initial design of the 37 mm filter cassette, the PIVEC is designed to maintain portability and mimic the in vivo deposition of particles from inhalation. The PIVEC is significantly smaller than current ALI exposu...
Ujawnienia
The affiliation of the authors is as shown on the cover page. The authors are financially supported by Virginia Commonwealth University, where the work was completed in Richmond, VA. The authors have sole responsibility for the writing and content of this paper. The authors declare that there are no competing interests.
Podziękowania
The authors would like to thank Boris Solomonov and the Virginia Commonwealth Innovation Machine Shop for help with rapid prototyping the device. The authors would also like to thank Cristian Romero-Fuentes of the Lewinski Group, Dr. Vitaliy Avrutin, Dr. Dmitry Pestov, and the Virginia Commonwealth Nanomaterials Core Characterization Facility for their help with particle characterization. This work was supported by startup funds provided to Dr. Lewinski by the College of Engineering at Virginia Commonwealth University.
Materiały
| Name | Company | Catalog Number | Comments |
| Scanning mobility particle sizer (SMPS) | TSI, Inc. | 3910 | NanoSMPS |
| Optical particle sizer (OPS) | TSI, Inc. | 3330 | |
| Stainless Steel Pipe, 4" Long | McMaster-Carr | 4830K116 | Standard-Wall 304/304L, Threaded on Both Ends, 1/8 Pipe Size |
| Brass Ball Valve with Lever Handle | McMaster-Carr | 4112T12 | Compact High-Pressure Rating, 1/8 NPT Female |
| Steel Pipe, 2" Long | McMaster-Carr | 7753K121 | Standard Wall, Threaded on One End, 1/8 Pipe Size |
| HEPA filter | GE Healthcare | 09-744-12 | HEPA-Cap Disposable Air Filtration Capsule |
| Vacuum Generator | PISCO USA | VCH10-018C | |
| PIVEC | VCU | For design please contact authors | |
| Resistive heater | |||
| 1/4" barbed connectors | Zefon International, Inc. | 459743 | |
| Porous tubing | Scientific Commodities, Inc. | BB2062-1814A | Hydrophilic 10 um pores |
| Battery power bank | |||
| Cell culture insert | Fisherbrand | 353095 | 24 well plate insert |
| Filter Forceps | Fisherbrand | 09-753-50 | |
| Transfer Pipette | ThermoScientific | 13-711-27 | |
| Glass Fiber Filters | SKC | 225-7 | Binder-Free Type AE Filter 37 MM 1.00 um pore |
| Ultra Micro Balance | A&D | BM-22 | Housed in environmental chamber |
| 37 mm filter cassette | SKC | 225-3250 | Filter Cassette Blank, 37 mm, Clear Styrene |
| Variable flow vacuum pump | SKC | 220-5000TC | AirChek TOUCH, 5 to 5000 mL/min |
| Copper Particles | U.S. Research Materials, Inc. | US1090 | 40 nm |
| Copper Particles | U.S. Research Materials, Inc. | US1088 | 100 nm |
| Copper Particles | U.S. Research Materials, Inc. | US1117M | 800 nm |
Odniesienia
- Lewinski, N. A., Secondo, L. E., Ferri, J. K. Enabling Real-Time Hazard Assessment at the Workplace Enabling Real-Time Hazard Assessment at the Workplace. 14th Global Congress on Process Safety. , 1-9 (2018).
- Bakand, S., Winder, C., Khalil, C., Hayes, A. Toxicity assessment of industrial chemicals and airborne contaminants: transition from in vivo to in vitro test methods: a review. Inhalation Toxicology. 17, 775-787 (2005).
- Bakand, S., Hayes, A. Troubleshooting methods for toxicity testing of airborne chemicals in vitro. Journal of Pharmacological and Toxicological Methods. 61 (2), 76-85 (2010).
- Lenz, A. G., et al. A dose-controlled system for air-liquid interface cell exposure and application to zinc oxide nanoparticles. Particle and Fibre Toxicology. 6, 32 (2009).
- de Bruijne, K., et al. Design and testing of Electrostatic Aerosol in Vitro Exposure System (EAVES): an alternative exposure system for particles. Inhalation Toxicology. 21, 91-101 (2009).
- Asimakopoulou, A., Daskalos, E., Lewinski, N., Riediker, M., Papaioannou, E., Konstandopoulos, A. G. Development of a dose-controlled multiculture cell exposure chamber for efficient delivery of airborne and engineered nanoparticles. Journal of Physics: Conference. 429, 1-10 (2013).
- Grigg, J., et al. DNA damage of macrophages at an air-tissue interface induced by metal nanoparticles Macrophage. Nanotoxicology. 3 (4), 348-354 (2009).
- Aufderheide, M., Knebel, J. W., Ritter, D. An improved in vitro model for testing the pulmonary toxicity of complex mixtures such as cigarette smoke. Experimental and Toxicologic Pathology. 55, 51-57 (2003).
- Aufderheide, M., Halter, B., Möhle, N., Hochrainer, D. The CULTEX RFS: A comprehensive technical approach for the in vitro exposure of airway epithelial cells to the particulate matter at the air-liquid interface. BioMed Research International. 2013 (1), 1-15 (2013).
- Tippe, A., Heinzmann, U., Roth, C. Deposition of fine and ultrafine aerosol particles during exposure at the air/cell interface. Journal of Aerosol Science. 33, 207-218 (2002).
- Savi, M., et al. A novel exposure system for the efficient and controlled deposition of aerosol particles onto cell cultures. Environmental Science and Technology. 42, 5667-5674 (2008).
- Fröhlich, E., et al. Comparison of two in vitro systems to assess cellular effects of nanoparticles-containing aerosols. Toxicology in Vitro. 27, 409-417 (2013).
- Frijns, E., et al. A Novel Exposure System Termed NAVETTA for in Vitro Laminar Flow Electrodeposition of Nanoaerosol and Evaluation of Immune Effects in Human Lung Reporter Cells. Environmental Science and Technology. 51 (9), 5259-5269 (2017).
- Vincent, J. H. . Aerosol Science for Industrial Hygienists. , (1995).
- Fujitani, Y., Sugaya, Y., Hashiguchi, M., Furuyama, A., Hirano, S., Takami, A. Particle deposition efficiency at air-liquid interface of a cell exposure chamber. Journal of Aerosol Science. 81, 90-99 (2015).
- Elihn, K., Cronholm, P., Karlsson, H. L., Midander, K., Odnevall Wallinder, I., Möller, L. Cellular Dose of Partly Soluble Cu Particle Aerosols at the Air-Liquid Interface Using an. In Vitro Lung Cell Exposure System. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 26 (2), 84-93 (2013).
- . . Aerosols Handbook Measurement, Dosimetry, and Health Effects. , (2005).
- de Souza Carvalho, C., Daum, N., Lehr, C. M. Carrier interactions with the biological barriers of the lung: Advanced in vitro models and challenges for pulmonary drug delivery. Advanced Drug Delivery Reviews. 75, 129-140 (2014).
- Fattal, E., Grabowski, N., Mura, S., Vergnaud, J., Tsapis, N., Hillaireau, H. Lung Toxicity of Biodegradable Nanoparticles. Journal of Biomedical Nanotechnology. 10 (10), 2852-2864 (2014).
- Klein, S. G., Serchi, T., Hoffmann, L., Blömeke, B., Gutleb, A. C. An improved 3D tetraculture system mimicking the cellular organisation at the alveolar barrier to study the potential toxic effects of particles on the lung. Particle and Fibre Toxicology. 10, 31 (2013).
- Oberdörster, G., et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Particle and Fibre Toxicology. 2, 8 (2005).
- Secondo, L. E., Liu, N. J., Lewinski, N. A. Methodological considerations when conducting in vitro, air-liquid interface exposures to engineered nanoparticle aerosols. Critical Reviews in Toxicology. , 1-32 (2016).
- Sayes, C. M., Reed, K. L., Warheit, D. B. Assessing toxicology of fine and nanoparticles: Comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicological Sciences. 97 (1), 163-180 (2007).
- Maier, K. L., et al. Health effects of ambient particulate matter--biological mechanisms and inflammatory responses to in vitro and in vivo particle exposures. Inhalation Toxicology. 20 (May 2007), 319-337 (2008).
- Cohen, J. M., Teeguarden, J. G., Demokritou, P. An integrated approach for the in vitro dosimetry of engineered nanomaterials. Particle and Fibre Toxicology. 11 (1), 20 (2014).
- Deloid, G., et al. Estimating the effective density of engineered nanomaterials for in vitro dosimetry. Nature Communications. 5, 3514 (2014).
- Pal, A. K., Bello, D., Cohen, J., Demokritou, P. Implications of in vitro dosimetry on toxicological ranking of low aspect ratio engineered nanomaterials. Nanotoxicology. , 1-15 (2015).
- Walkey, C., et al. Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano. 8 (3), 2439-2455 (2014).
- Raemy, D. O., et al. Effects of flame made zinc oxide particles in human lung cells - a comparison of aerosol and suspension exposures. Particle and Fibre Toxicology. 9 (1), 33 (2012).
- Holder, A. L., Lucas, D., Goth-goldstein, R., Koshland, C. P. Cellular response to diesel exhaust particles strongly depends on the exposure method. Toxicological Sciences. 103 (1), 108-115 (2008).
- Sanderson, P., et al. Characterisation of iron-rich atmospheric submicrometre particles in the roadside environment. Atmospheric Environment. 140. , 167-175 (2016).
- Burtscher, H. Physical characterization of particulate emissions from diesel engines: A review. Journal of Aerosol Science. 36 (7), 896-932 (2005).
- Ris, C. U.S. EPA health assessment for diesel engine exhaust: a review. Inhalation Toxicology. 19 (Suppl. 1), 229-239 (2007).
- Jie, Y., Isa, Z. M., Jie, X., Ju, Z. L., Ismail, N. H. Urban vs. Rural Factors That Affect Adult Asthma. Reviews of Environmental Contamination and Toxicology. 226, (2013).
- Wiemann, M., Vennemann, A., Sauer, U. G., Wiench, K., Ma-Hock, L., Landsiedel, R. An in vitro alveolar macrophage assay for predicting the short-term inhalation toxicity of nanomaterials. Journal of Nanobiotechnology. 14 (1), 16 (2016).
- Kenny, L. C., et al. A collaborative european study of personal inhalable aerosol sampler performance. Annals of Occupational Hygiene. 41 (2), 135-153 (1997).
- Tiwari, A. J., Fields, C. G., Marr, L. C. A Cost-Effective Method of Aerosolizing Dry Powdered Nanoparticles. Aerosol Science and Technology. 47 (11), 1267-1275 (2013).
- Blank, F., Rothen-Rutishauser, B. M., Schurch, S., Gehr, P. An Optimized In Vitro Model of the Respiratory Tract Wall to Study Particle Cell Interactions. Journal of Aerosol Medicine. 19 (3), 392-405 (2006).
- Laboratory, N. C. . NCL Method GTA-2 HEP G2 Hepatocarcinoma Cytotoxicity Assay. (November), 1-9 (2015).
- Kim, J. S., Peters, T. M., O'Shaughnessy, P. T., Adamcakova-Dodd, A., Thorne, P. S. Validation of an in vitro exposure system for toxicity assessment of air-delivered nanomaterials. Toxicology in Vitro. 27 (1), 164-173 (2013).
- Mertes, P., et al. A compact and portable deposition chamber to study nanoparticles in air-exposed tissue. Journal of aerosol medicine and pulmonary drug delivery. 26, 228-235 (2013).
- Panas, A., et al. Silica nanoparticles are less toxic to human lung cells when deposited at the air-liquid interface compared to conventional submerged exposure. Beilstein Journal of Nanotechnology. 5, 1590-1602 (2014).
- Zavala, J., et al. Regulating temperature and relative humidity in air-liquid interface in vitro systems eliminates cytotoxicity resulting from control air exposures. Toxicology Research. 6, 448-459 (2017).
- Jing, X., Park, J. H., Peters, T. M., Thorne, P. S. Toxicity of copper oxide nanoparticles in lung epithelial cells exposed at the air - liquid interface compared with in vivo assessment. TOXICOLOGY IN VITRO. 29 (3), 502-511 (2015).
- Bitterle, E., et al. Dose-controlled exposure of A549 epithelial cells at the air-liquid interface to airborne ultrafine carbonaceous particles. Chemosphere. 65, 1784-1790 (2006).
- Steinritz, D., et al. Use of the Cultex Radial Flow System as an in vitro exposure method to assess acute pulmonary toxicity of fine dusts and nanoparticles with special focus on the intra- and inter-laboratory reproducibility. Chemico-Biological Interactions. 206 (3), 479-490 (2013).
- Cronholm, P., et al. Intracellular uptake and toxicity of Ag and CuO nanoparticles: A comparison between nanoparticles and their corresponding metal ions. Small. 9 (7), 970-982 (2013).
- Cronholm, P., Midander, K., Karlsson, H. L., Elihn, K., Wallinder, I. O., Möller, L. Effect of sonication and serum proteins on copper release from copper nanoparticles and the toxicity towards lung epithelial cells. Nanotoxicology. 5 (2), 269-281 (2011).
- Midander, K., et al. Surface characteristics, copper release, and toxicity of nano- and micrometer-sized copper and copper(ll) oxide particles: A cross-disciplinary study. Small. 5 (3), 389-399 (2009).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone