Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Chemical-Induced Skin Carcinogenesis Model Using Dimethylbenz[a]Anthracene and 12-O-Tetradecanoyl Phorbol-13-Acetate (DMBA-TPA)
W tym Artykule
Podsumowanie
Two-stage skin carcinogenesis is induced by two topically applied chemicals. A mutagen 7,12-dimethylbenz[a]anthracene) causes mutations in the epidermal cells and a continuous application of general growth stimulator 12-O-tetradecanoyl phorbol-13-acetate accelerates skin papilloma formation.
Streszczenie
Cancer is one of the most devastating human diseases. Experimental cancer models are important to gain insight into the complex interplay of different cell types and genes in promoting tumor progression and to provide a platform for testing the efficacy of different therapeutic approaches. One of the most commonly used experimental inflammatory cancer models is the DMBA-TPA two-stage skin carcinogenesis model. Tumor formation is induced in this model by the topical application of two different chemicals, 7,12-dimethylbenz[a]anthracene (DMBA) and 12-O-tetradecanoyl phorbol-13-acetate (TPA), that together cause papilloma formation in the skin. As the primary outcome is papilloma formation in the skin, the model is an ideal, reliable, and reproducible way to address both tumor initiation (tumor-free survival) and tumor progression (number and size of visible tumors). The effects of the DMBA-TPA treatment are transmitted via an inflammatory mechanism, which makes this model especially suitable for studying the role of the immune system in tumor formation. However, this model is restricted to the skin and other surfaces where the chemicals can be applied on. A detailed protocol is provided in this article to use the model successfully.
Wprowadzenie
Cancer is one of the leading causes of death in the world. Therefore, there is a demand to develop reliable experimental disease models to obtain a better understanding of the disease as well as to explore potential therapeutic approaches. One of the most commonly used experimental in vivo models to study skin cancer development is the chemically induced two-stage skin carcinogenesis model1,2. The model provides a tool to study tumor initiation, promotion, and progression in addition to specific events such as immune cell infiltration and angiogenesis.
To use the two-stage skin carcinogenesis model, the back skin of mice is treated with two different chemicals that together induce tumor formation. The model is initiated with a low dose of the mutagen, DMBA, followed by prolonged exposure to the tumor promoter, TPA3 (Figure 1). DMBA mutates DNA randomly by forming covalent adducts with the DNA of epidermal cells and primary keratinocyte stem cells4,5,6,7. Some of these random mutations take place in a proto-oncogene, such as Hras1 (mutations in Kras and Nras are also detected) and the conversion of proto-oncogenes to oncogenes drives the tumor formation under proper stimuli. TPA, in turn, is the most commonly used tumor growth-promoting agent. Its molecular target is protein kinase C (PKC)8. TPA also activates Wnt/β-catenin signaling that is crucial for tumor formation in the model9. Repeated and prolonged exposure to the promoting agent leads to enhanced cell signaling, increased production of growth factors, and a local inflammatory reaction, which are evident due to increased DNA synthesis and inflammatory cell infiltration in the treated skin.
The key inflammatory mediators in the DMBA-TPA model have been identified10. Interleukin-17A (IL-17A) is known to be particularly tumorigenic in the DMBA-TPA model11,12. It works in synergy with interleukin 6 (IL-6) and participates in macrophage and neutrophil recruitment13,14. In addition, CD4+ T cells and neutrophils have been shown to be tumorigenic in the DMBA-TPA model. Finally, macrophages can also promote tumorigenesis in the model15,16,17.
During the promotion phase, the cell proliferation of the mutated cells is enhanced and a sustained hyperplasia of the epidermis is maintained1. This leads to papilloma development in the skin in 10–20 weeks, after which the papillomas start to convert to malignant tumors, squamous cell carcinomas (SCCs)2. However, less than 10% of the papillomas progress to malignancy, although this percentage also depends on the genetic background of the mice2,18. For decades it was not known what type of cells were initially mutated in the tumors leading to malignancy, even though some studies had reported clearly distinct features in the malignant tumors when compared to benign papillomas19,20. However, recent studies have greatly increased our understanding on the clonal origin of tumor formation in the DMBA-TPA model21.22.23. It was demonstrated that both bone marrow-derived epithelial cells and hair follicle stem cells contribute to the tumor formation22. Stage-specific lineage tracing studies have unveiled that benign papillomas are of monoclonal origin, but they recruit new epithelial cell populations21,23. However, only one of the cell clones functions as a driver for carcinogenesis; it contains an Hras mutation23. The progression to carcinoma formation is associated with a clonal sweep23.
The carcinogen DMBA initiates the papilloma formation and TPA promotes tumor growth. Hence, the tumor initiation can be studied separately from the promotion by interrupting the experiment before the TPA treatment period. As the tumor progression is studied weekly it offers a great opportunity for detailed tumor growth analysis throughout the study. Because the tumors are generated by external chemicals, an oncogenic mutation in the germline is unnecessary. Thus, studying the effects of a genetic background (e.g., knockout/transgene vs. wild type) on tumorigenesis is straightforward2. In sum, the DMBA/TPA skin cancer model is a particularly useful approach for studying the role of the immune system in tumor progression as well as for the evaluation of tumor initiation and promotion steps independently or interdependently.
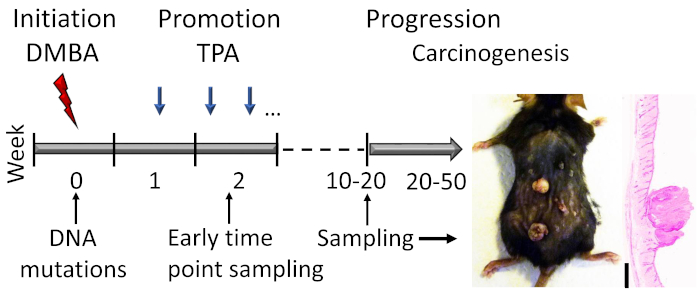
Figure 1: DMBA-TPA-induced skin carcinogenesis model outline. The carcinogen DMBA is topically applied to induce DNA mutations in the initiation phase of the model. The growth-promoting agent TPA is administered 2x a week to enhance cell proliferation during the promotion phase, leading to the development of papillomas in the skin. Animals are sacrificed after the papilloma response reaches a plateau, usually within weeks 15–20, depending on the genetic background of the mice. A small proportion of the papillomas can further develop into SCCs within 20–50 weeks. To study early events in the initiation and early promotion phase, samples can be collected (e.g., shortly after the second TPA application). A representative photograph and hematoxylin and eosin stained cross section of papillomas on a C57BL/6 mouse skin after 19 weeks of treatment are shown. Scale bar = 0.1 mm. Please click here to view a larger version of this figure.
Protokół
The protocol described here has been approved by the National Animal Ethics Committee of Finland (protocol number ESAVI/23659/2018).
1. Experimental Animals, Reagents, and Equipment
- Use age and sex-matched mice. Start the study at 7–9 weeks of age, because the skin in most mice is in telogen (the resting phase) around that age2.
- Observe the behavior of the animals during the study and if they fight, which often happens with males, house them separately. Fights may cause cuts in the skin, which promote tumor formation. Female mice are preferred due to their less aggressive behavior. The typical experimental group size varies between 8–20 animals per group24,25,26,27.
NOTE: Power calculations based on the biological variance seen in earlier studies help to choose a sufficiently large group size. The strains used as examples in this article include Balb/c and C57BL/6. However, many other mouse strains such as SENCAR and FVB have been used with the DMBA-TPA-model, as well as Wistar and Sprague-Dawley rats2,28,29. A license from the national or local committee of animal work is needed before the initiation of the study. In addition to general welfare considerations, the model-specific endpoints are typically squamous cell carcinoma (SCC) and an infection of the skin. Scratches in the skin due to itching after application of the tumorigenic chemicals in acetone is typical but otherwise the animals should show no signs of discomfort. Weighing regularly (e.g., 2x a month) helps evaluate the animals' welfare. - Use the DMBA and TPA, both diluted in acetone. The working concentration of DMBA is 250 g/L. A dose for one animal is 50 µg of DMBA in 200 µL of acetone. The stock concentration of TPA is 125 g/L and the treatment concentration 25 g/L. A TPA dose for one animal is 5 µg in 200 µL of acetone.
CAUTION: DMBA is harmful if swallowed and may cause cancer. Acetone evaporates rapidly and is flammable. It may cause dizziness and irritate eyes. Use a breathing mask and/or work under a vacuum flow. Change gloves after handling any of these chemicals. Individually ventilated cages prevent the spread of the chemicals during housing of the mice. After application, gather the pipette tips used for handling the DMBA and dispose as dangerous waste.
NOTE: The DMBA must be protected from light. The diluted TPA is stored in -20 °C, preferably protected from light. - Procure the following equipment: a scale, an ordinary shaver for the fur, pipettes and tips of an appropriate size, an ordinary ruler, a digital camera, a notepad and a pen or a computer for recording the papillomas, an inhaled anesthesia system or a mouse restrainer with an opening on the back top, and a carbon dioxide narcosis system for sacrificing mice.
2. Skin Papilloma Induction and Promotion
- Shave the back skin and weigh the animal. Later, shave the skin whenever needed but not at the time of the chemical exposure.
NOTE: Be careful when shaving around the papillomas and avoid making any cuts on the skin. Weigh each animal every 2 weeks to notice any potential weight loss. - Apply 50 µg of DMBA in 200 µL of acetone topically on the shaved area using a pipette 48 h after shaving the fur. If needed, restrain the animal using light inhalation anesthesia or a mouse restrainer.
- After 7 days, give the first TPA dose. Apply 5 µg of TPA in 200 µL of acetone topically with a pipette 2x a week, preferably Monday and Thursday or Tuesday and Friday.
- Count, record, and photograph the papillomas every week. A palpable mass greater than 1 mm in diameter is considered a papilloma if it stays longer than 1 week. Mark each individual papilloma on a map and list its size every week. Store digital photographs.
3. Animal Sacrifice and Sample Collection
- Continue the treatment until the tumor response reaches a plateau. Generally, the papilloma burden is expected to increase 10–20 weeks after the initiation, depending on the mouse strain used. The plateau is expected in 15–20 weeks. A small proportion of the papillomas (under 3%) can develop into SCCs within 20–50 weeks2.
- Sacrifice the animals 24 h after the last TPA application. Use carbon dioxide narcosis with cervical dislocation or another suitable method.
- Depending on the research question, gather appropriate sample material from the animals30,31.
- For example, take blood samples before sacrifice and separate the plasma.
- Cut pieces of the skin for immunohistochemistry (IHC) staining (e.g., hematoxylin and eosin, proliferating or inflammatory cells).
- Use biopsy punches to collect skin pieces with either papilloma tissue or non-papilloma (treated) skin for gene expression (e.g., qPCR) and/or protein analyses (e.g., Western blot or ELISA).
- Collect a piece of the spleen and skin-draining lymph nodes for flow cytometry analysis if more detailed analysis of the immune cell populations is desired. You may also separate epidermal and dermal layers for further analyses.
4. Statistics
- Draw a Kaplan-Meier survival curve of the papilloma-free time and use the Mantel-Cox log-rank test for the survival. Draw a linear curve of the number of papillomas per week. Because this is count data, use a nonlinear regression model. Consult a statistician if needed.
Wyniki
The main outcome is the survival (i.e., papilloma free) time between the treatment or genotype groups. The secondary outcome is the number of papillomas per week in each group (Figure 2). The expected results are a statistically significant difference in the papilloma free time and in the number of papillomas between the experimental (two or more) groups. It is recommended to count the number of papillomas and draw a curve during the promotion (TPA) phase to get an idea of the differences be...
Dyskusje
DMBA-TPA-induced skin cancer is one of the most commonly used cancer models because it is highly reproducible and provides information on tumor progression from initiation to malignancy. The key outcome measure, papilloma formation, is easily and reliably quantitative. The model addresses both tumor initiation (tumor-free survival) and progression (tumor numbers and sizes) simultaneously. The model is suitable for studying different compounds, such as potential therapeutics, and the effects of individual genes on tumor p...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This work was funded by the Academy of Finland (grants 25013080481 and 25013142041 (I.J.), 286377 and 295814 (M.P.), 287907 (T.J.)), Päivikki and Sakari Sohlberg Foundation (M.P., T.J.), Finnish Medical Foundation (T.P.), The Competitive State Research Financing of the Expert Responsibility Area of Tampere University Hospital (grant 9V049 and 9X044 (M.P.), 9X011 and 9V010 (T.J.)), The Competitive State Research Financing of the Expert Responsibility Area of Fimlab Laboratories (grant X51409 (I.J.)), Tays Support Foundation (I.J., M.P., T.J.), Tampere Tuberculosis Foundation (I.J., M.P., T.J.), the Finnish Cultural Foundation (M.V.), the Paulo Foundation (T.P.), Cancer Society of Finland (M.P.), and the Emil Aaltonen Foundation (T.P.).
Materiały
| Name | Company | Catalog Number | Comments |
| 1000 ul RPT XL Graduated Filter Tip (Sterile), Refill | Starlab | S1182-1730-C | |
| 300 ul RTP Graduated Filter Tip (sterile), Refill | Starlab | S1180-9710-C | |
| 7,12-Dimethylbenz[a]anthracene (DMBA) | Sigma | D3254-100MG | Harmful if swallowed and may cause cancer. Store protected from light. |
| Acetone | Sigma | 1000141011 | Evaporates rapidly and is inflammable. |
| Attane vet 1000 mg/g | Piramal Critical Care Limited | Liquid isoflurane for inhalation | |
| Battery-Operated Clipper Isis | Albert Kerlb GmbH | GT421 | For shaving the fur |
| CONTRAfluran-Restgasfilter | ZeoSys GmbH | For anesthesia | |
| Linex Nature N1030 Ruler 30 cm | Staples Business Advantage | 60383 | For measuring papillomas |
| Medium CO2 Chamber 300 x 200 x 200mm - Red | VetTech Solutions Ltd | AN045AR | For sacrifice |
| Mekasoft | Mekalasi | 23008 | Table cover |
| Mice (Balb/c JRj) | Janvier labs | Other strains also possible | |
| Mice (C57BL/6JRj) | Janvier labs | Other strains also possible | |
| Panasonic Lumix DMC-FS5 Digital Camera | Panasonic | ||
| Paraformaldehyde | Merck | 30525-89-4 | For histology samples |
| Phorbol 12-myristate 13-acetate aka 12-Otetradecanoylphorbol-13-acetate (TPA) | Enzo | BML-PE160-0001 | |
| Precision balance PLJ-C/PLJ-G | KERN & SOHN GmbH | PLJ 600-3CM | |
| Pre-Set CO2 System-2 Chamber-S/S Housing | VetTech Solutions Ltd | AN044BX | For sacrifice |
| RNAlater | Qiagen | 76104 | For nucleic acid samples |
| Tacta pipette 100-1000 ul | Sartorius | LH-729070 | |
| Tacta pipette 20-200 ul | Sartorius | LH-729060 | |
| UNO Anaesthetic Key Filler | Scintica instrumentation inc. | For anesthesia | |
| UNO Face Mask for Mouse | Scintica instrumentation inc. | For anesthesia | |
| UNO FM2200 Flowmeter | Scintica instrumentation inc. | For anesthesia | |
| UNO Gas Exhaust Unit | Scintica instrumentation inc. | For anesthesia | |
| UNO Induction Box | Scintica instrumentation inc. | For anesthesia | |
| UNO200VAP Vaporizer | Scintica instrumentation inc. | For anesthesia |
Odniesienia
- DiGiovanni, J. Multistage carcinogenesis in mouse skin. Pharmacology & Therapeutics. 54 (1), 63-128 (1992).
- Abel, E. L., Angel, J. M., Kiguchi, K., DiGiovanni, J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nature Protocols. 4 (9), 1350-1362 (2009).
- Perez-Losada, J., Balmain, A. Stem-cell hierarchy in skin cancer. Nature Reviews. Cancer. 3 (6), 434-443 (2003).
- Bonham, K., et al. Activation of the cellular Harvey ras gene in mouse skin tumors initiated with urethane. Molecular Carcinogenesis. 2 (1), 34-39 (1989).
- Quintanilla, M., Brown, K., Ramsden, M., Balmain, A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 322 (6074), 78-80 (1986).
- Nelson, M. A., Futscher, B. W., Kinsella, T., Wymer, J., Bowden, G. T. Detection of mutant Ha-ras genes in chemically initiated mouse skin epidermis before the development of benign tumors. Proceedings of the National Academy of Sciences of the United States of America. 89 (14), 6398-6402 (1992).
- Morris, R. J. A perspective on keratinocyte stem cells as targets for skin carcinogenesis. Differentiation. 72 (8), 381-386 (2004).
- Chung, Y. W., Kim, H. K., Kim, I. Y., Yim, M. B., Chock, P. B. Dual function of protein kinase C (PKC) in 12-O-tetradecanoylphorbol-13-acetate (TPA)-inducec manganese superoxide dismutase (MnSOD) expression: activation of CREB and FOXO3a by PKC-alpha phosphorylation and by PKC-mediated inactivation of Akt, respectively. The Journal of Biological Chemistry. 286 (34), 29681-29690 (2011).
- Su, Z., et al. Tumor promoter TPA activates Wnt/β-catenin signaling in a casein kinase 1-dependent manner. Proceedings of the National Academy of Sciences of the United States of America. 115 (32), 7522-7531 (2018).
- Swann, J. B., et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 105 (2), 652-656 (2008).
- Wang, L., Yi, T., Zhang, W., Pardoll, D. M., Yu, H. IL-17 enhances tumor development in carcinogen-induced skin cancer. Cancer Research. 70 (24), 10112-10120 (2010).
- He, D., et al. IL-17 mediated inflammation promotes tumor growth and progression in the skin. PLoS One. 7 (2), 32126 (2012).
- Roussel, L., et al. IL-17 promotes p38 MAPDK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammation. Journal of Immunology. 184 (8), 4531-4537 (2010).
- Wanqiu, H., Young-Hee, J., Hyun, S. K., Byung, S. K. Interleukin-6 (IL-6) and IL-17 synergistically promote viral persistence by inhibition cellular apoptosis and cytotoxic T cell function. Journal of Virology. 88 (15), 8479-8489 (2014).
- Yusuf, N., et al. Antagonistic roles of CD4+ and CD8+ T-cells in 7,12-dimethylbenz(a)anthracene cutaneous carcinogenesis. Cancer Research. 68 (10), 3924-3930 (2008).
- Gong, L., et al. Promoting effect of neutrophils on lung tumorigenesis is mediated by CXCR2 and neutrophil elastase. Molecular Cancer. 12 (1), 154 (2013).
- Vestweber, D., Wessel, F., Nottebaum, A. F. Similarities and differences in the regulation of leukocyte extravasation and vascular permeability. Seminars in Immunopathology. 36 (2), 177-192 (2014).
- Woodworth, C. D., et al. Strain-dependent differences in malignant conversion of mouse skin tumors is an inherent property of the epidermal keratinocyte. Carcinogenesis. 25 (9), 1771-1778 (2004).
- Tennenbaum, T., et al. The suprabasal expression of alpha 6 beta 4 integrin is associated with a high risk for malignant progression in mouse skin carcinogenesis. Cancer Research. 53 (20), 4803-4810 (1993).
- Hennings, H., Shores, R., Mitchell, P., Spangler, E. F., Yuspa, S. H. Induction of papillomas with a high probability of conversion to malignancy. Carcinogenesis. 6 (11), 1607-1610 (1985).
- Auto, Y., et al. Time-Series Analysis of Tumorigenesis in a Murine Skin Carcinogenesis Model. Scientific Reports. 8 (1), 12994 (2018).
- Park, H., et al. Bone marrow-derived epithelial cells and hair follicle stem cells contribute to development of chronic cutaneous neoplasms. Nature Communications. 9 (1), 5293 (2018).
- Reeves, M. Q., Kandyba, E., Harris, S., Del Rosario, R., Balmain, A. Multicolour lineage tracing reveals clonal dynamics of squamous carcinoma evolution from initiation to metastasis. Nature Cell Biology. 20 (6), 699-709 (2018).
- Dao, V., et al. Prevention of carcinogen and inflammation-induced dermal cancer by oral rapamycin includes reducing genetic damage. Cancer Prevention Research. 5, 400-409 (2015).
- Yeong, L. T., Abdul Hamid, R., Saiful Yazan, L., Khaza’ai, H., Mohtarrudin, N. Low dose triterpene-quinone fraction from Ardisia crispa root precludes chemical-induced mouse skin tumor promotion. BMC Complementary and Alternative Medicine. 15 (1), 431 (2015).
- Kong, Y. H., Xu, S. P. Salidroside prevents skin carcinogenesis induced by DMBA/TPA in a mouse model through suppression of inflammation and promotion of apoptosis. Oncology Reports. 39 (6), 2513-2526 (2018).
- Jung, M., Bu, S. Y., Tak, K. H., Park, J. E., Kim, E. Anticarcinogenic effect of quercetin by inhibition of insulin-like growth factor (IGF)-1 signaling in mouse skin cancer. Nutrition Research and Practice. 7 (6), 439-445 (2013).
- Hu, Y. Q., Wang, J., Wu, J. H. Administration of resveratrol enhances cell-cycle arrest followed by apoptosis in DMBA-induced skin carcinogenesis in male Wistar rats. European review for medical and pharmacological sciences. 13, 2935-2946 (2016).
- Schweizer, J., Loehrke, H., Hesse, B., Goerttler, K. 7,12-Dimethylbenz[a]anthracene/12-O-tetradecanoyl-phorbol-13-acetate-mediated skin tumor initiation and promotion in male Sprague-Dawley rats. Carcinogenesis. 3 (7), 785-789 (1982).
- Vähätupa, M., et al. T-cell-expressed proprotein convertase FURIN inhibits DMBA/TPA-induced skin cancer development. Oncoimmunology. 5 (12), 1245266 (2016).
- May, U., et al. Resistance of R-Ras knockout mice to skin tumour induction. Scientific Reports. 5, 11663 (2015).
- Krajewska, M., et al. Image analysis algorithms for immunohistochemical assessment of cell death events and fibrosis in tissue sections. The Journal of Histochemistry and Cytochemistry. 57 (7), 649-663 (2009).
- Järvinen, T. A., Ruoslahti, E. Target-seeking antifibrotic compound enhances wound healing and suppresses scar formation in mice. Proceedings of the National Academy of Sciences of the United States of America. 107 (50), 21671-21676 (2010).
- Schwarz, M., Münzel, P. A., Braeuning, A. Non-melanoma skin cancer in mouse and man. Archives of Toxicology. 87 (5), 783-798 (2013).
- Slaga, T. J. SENCAR mouse skin tumorigenesis model versus other strains and stocks of mice. Environmental Health Perspectives. 68, 27-32 (1986).
- Goerttler, K., Loehrke, H., Schweizer, J., Hesse, B. Systemic two-stage carcinogenesis in the epithelium of the forestomach of mice using 7,12-dimethylbenz(a)anthracene as initiator and the phorbol ester 12-O-tetradecanoylphorbol-13-acetate as promoter. Cancer Research. 39 (4), 1293-1297 (1979).
- Topping, D. C., Nettesheim, P. Promotion-like enhancement of tracheal carcinogenesis in rats by 12-O-tetradecanoylphorbol-13-acetate. Cancer Research. 40, 4352-4355 (1980).
- Wille, J. J. Circadian rhythm of tumor promotion in the two-stage model of mouse tumorigenesis. Cancer Letters. 190 (2), 143-149 (2003).
- Lee, Y. S., et al. Inhibition of skin carcinogenesis by suppression of NF-κB dependent ITGAV and TIMP-1 expression in IL32γ overexpressed condition. Journal of Experimental & Clinical Cancer Research. 37 (1), 293 (2018).
- Kiss, A., et al. Cell type-specific p38δ targeting reveals a context-, stage-, and sex-dependent regulation of skin carcinogenesis. International Journal of Molecular Sciences. 20 (7), 1532 (2019).
- Tomo-o, I., et al. Positron emission tomography imaging of DMBA/TPA mouse skin multi-step tumorigenesis. Molecular Oncology. 4 (2), 119-125 (2010).
- Mantovani, A., Allavena, P., Sica, A., Balkwill, F. Cancer-related inflammation. Nature. 454 (7203), 436-444 (2008).
- Crusz, S. M., Balkwill, F. R. Inflammation and cancer: advances and new agents. Nature Reviews. Clinical Oncology. 12 (10), 584-596 (2015).
- Hennings, L., et al. Malignant conversion and metastasis of mouse skin tumors: a comparison of SENCAR and CD-1 mice. Environmental Health Perspectives. 68, 69-74 (1986).
- Gómez-Cuadrado, L., Tracey, N., Ma, R., Qian, B., Brunton, V. G. Mouse models of metastasis: progress and prospects. Disease Models & Mechanisms. 10 (9), 1061-1074 (2017).
- Ouhtit, A., Ananthaswamy, H. N. A model for UV-induction of skin cancer. Journal of Biomedicine and Biotechnology. 1 (1), 5-6 (2001).
- Day, C. -. P., Marchalik, R., Merlino, G., Michael, H. T. Mouse models of UV-induced melanoma: genetics, pathology, and clinical relevance. Laboratory Investigation. 97 (6), 698-705 (2017).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone