Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Fear Incubation Using an Extended Fear-Conditioning Protocol for Rats
W tym Artykule
Podsumowanie
We describe an extended fear-conditioning protocol that produces overtraining and fear incubation in rats. This protocol entails a single training session with 25 tone-shock pairings (i.e., overtraining) and a comparison of conditioned freezing responses during context and cue tests 48 h (short-term) and 6 weeks (long-term) after training.
Streszczenie
Emotional memory has been primarily studied with fear-conditioning paradigms. Fear conditioning is a form of learning through which individuals learn the relationships between aversive events and otherwise neutral stimuli. The most-widely utilized procedures for studying emotional memories entail fear conditioning in rats. In these tasks, the unconditioned stimulus (US) is a footshock presented once or several times across single or several sessions, and the conditioned response (CR) is freezing. In a version of these procedures, called cued fear conditioning, a tone (conditioned stimulus, CS) is paired with footshocks (US) during the training phase. During the first test, animals are exposed to the same context in which training took place, and freezing responses are tested in the absence of footshocks and tones (i.e., a context test). During the second test, freezing is measured when the context is changed (e.g., by manipulating the smell and walls of the experimental chamber) and the tone is presented in the absence of footshocks (i.e., a cue test). Most cued fear conditioning procedures entail few tone-shock pairings (e.g., 1-3 trials in a single session). There is a growing interest in less common versions involving an extensive number of pairings (i.e., overtraining) related to the long-lasting effect called fear incubation (i.e., fear responses increase over time without further exposure to aversive events or conditioned stimuli). Extended fear-conditioning tasks have been key to the understanding of fear incubation’s behavioral and neurobiological aspects, including its relationship with other psychological phenomena (e.g., post-traumatic stress disorder). Here, we describe an extended fear-conditioning protocol that produces overtraining and fear incubation in rats. This protocol entails a single training session with 25 tone-shock pairings (i.e., overtraining) and a comparison of conditioned freezing responses during context and cue tests 48 h (short-term) and 6 weeks (long-term) after training.
Wprowadzenie
Memory is a psychological process encompassing different phases: information acquisition, consolidation (allows for the stability of acquired information), and retrieval (evidence for the consolidation process)1. During the consolidation phase, the establishment of new synaptic connections and modification of pre-existing connections occur. This suggests the necessity for a period of time during which molecular and physiological events responsible for these changes occur1,2. These physiological or molecular changes vary whether the retrieved events are emotionally charged or not (i.e., emotional memory). For instance, research has shown that the lateral nucleus and basolateral amygdala complex are particularly relevant to emotional memory3,4,5.
Emotional memory phenomena have been primarily studied with fear conditioning paradigms5,6. Fear conditioning is a form of learning through which individuals learn the relationships between aversive events and otherwise neutral stimuli7. Fear conditioning paradigms produce molecular, cellular, and structural changes in the amygdala. In addition, fear conditioning modifies the connectivity of the hippocampus during the consolidation and retrieval processes of emotional memory.
One of the most commonly used procedures for studying fear memories is classical (Pavlovian) conditioning in rats. This procedure typically uses footshock (US) as the aversive stimulus, which is delivered once or several times across one or several sessions. The conditioned response (CR) of rats exposed to this procedure is freezing (i.e., “generalized immobility caused by a generalized tonic response of the animals’ skeletal musculature except those muscles used in breathing”7 ). This response could be assessed on two types of tests: context and cue tests. For the context test, the subject undergoes a given number of footshocks during the training session, and then is removed from the experimental chamber for a defined time. During the test, the subject is returned to the same context in which the training took place and different measures of freezing are collected in the absence of footshocks (e.g., duration, percentage or frequency of freezing episodes), and compared to baseline levels established during the training phase. For the second type of test, cue test, a stimulus (typically a tone) is paired with the footshocks during the training phase (i.e., conditional stimulus, CS). After training is completed, the animal is removed from the training context for a defined time and is subsequently placed in a modified context (e.g., a different experimental chamber that has different shapes of walls and different smell). The cue is then presented a given number of times, and freezing responses to the cue are measured and compared to baseline levels collected during training. The most common version of this paradigm uses 1 to 3 tone-shock pairings during a single training session, followed by context and cue tests conducted a number of hours or a few days later.
Other less frequently implemented fear conditioning procedures involve an extensive number of shock-cue pairings (i.e., trials), which have often been called overtraining procedures8. A growing interest in these tasks is related to their long-lasting and increased memory effects called fear incubation (i.e., conditioned fear responses increase over time in the absence of further exposure to aversive events or conditioned stimuli)9,10,11. An example of such overtraining procedures entails a training phase of 100 tone-shock pairings distributed across 10 sessions, followed by context and cue tests conducted 48 h and 30 days later11,12. To avoid extensive training spread across several days, Maren (1998) reported that overtraining could be established and optimized in a single session with 25 pairings8. The incubation effect is evidenced in significantly higher levels of conditioned fear in rats tested 31 days after training, as compared to rats tested 48 h after. Extended fear-conditioning tasks have been key for the understanding of behavioral and neurobiological aspects underlying fear incubation, including its relationship with other psychological phenomena (e.g., delayed-onset post-traumatic stress disorder)11,12,13.
Here, we describe an extended fear-conditioning protocol that induces overtraining and fear incubation in rats. Different to other paradigms that require several days of training11, the current protocol is focused on a single training session8. We used 25 tone-shock pairings to produce higher conditioned freezing responses during context and cue tests conducted 6 weeks after training, as compared to tests conducted 48 h after.
Access restricted. Please log in or start a trial to view this content.
Protokół
The following protocol was approved by the Institutional Animal Care and Use Committee of Fundación Universitaria Konrad Lorenz (IACUC-KL). The universal declaration of animal rights issued by International League of Animal Rights, Geneva, Switzerland (1989), and ethical principles of experimentation with animals issued by ICLAS were respected.
1. Subject preparation
- Select male adult Wistar rats (n = 12). House them in groups of four per cage for three days of acclimatization, prior to the beginning of the training and testing protocol. Provide rats with free access to water throughout the experiment. Control the room temperature between 20 °C to 25 °C, under a 12 h light-dark cycle (lights on at 07:00 h).
NOTE: Rat strains had shown differential performance during fear conditioning. For instance, Schaap et al. (2013) reported that Wistar and Lewis strains showed longer durations of freezing behavior compared with Fawn Hooded and Brown Norway rats12. Thus, differences in pain and thermal threshold should be assessed to adjust the intensity and duration of shocks. - Maintain rats at 85% of their free-feeding weights (normal weight between 350-400 g) by giving restricted food access at the same hour every day. Weigh rats every day at the same hour during the light cycle. Calculate the ad lib weight (100% weight) for three days before the start of extended fear-conditioning training.
NOTE: Animals used in the present experiment were tested on additional instrumental tests that are not reported in here. Food deprivation was required for those additional tests. This procedural variation is assumed as likely to expand the scope of the present procedure, as it suggests the potential for instrumental-fear combined tests. However, studies using only fear conditioning tests will not require food deprivation. - Randomly assign subjects to one of the following groups: emotional testing 6 weeks after training (n = 6); emotional testing 48 h after training (n = 6).
- Perform training and tests at similar hours, during the light phase of dark-light cycle. Assign the animals to the same experimental chamber and maintain the same order of animals during training and testing.
NOTE: An additional control that could be implemented is counterbalancing the order of animals during training and testing phases. We recommend using this technique when multiples groups are assessed, or different tasks are applied across experiments, to reduce a possible effect of task-order on behavior.
2. Apparatus setting and shock calibration
- Clean all the internal surfaces of the experimental chamber and stainless-steel grid floor with 10% ethanol. Repeat before testing each animal.
- Connect the equipment to a computer using a USB cable and start the freezing detection system equipment: the CPU, the controlling cabinet, the infrared light, the aversive stimulator/scrambler, and the shock-intensity calibrator.
NOTE: Although this protocol was executed using commercially available instruments (Table of Materials), equipment and software of different brands can be used. The apparatus consists of an internal acrylic square chamber (29.53 cm x 23.5 cm x 20.96 cm, called the experimental chamber) embedded in a wooden box covered with plastic formic. The external doors allow the isolation of sound, noise or light (attenuating box doors). The camera is located laterally in the internal part of the external door. The internal acrylic box with floor metal grids (36 stainless-steel rods, each one of 3 mm diameter and spaced 8 mm, center to center) allows footshock delivery. In one of the internal-lateral walls, a speaker is located 6 cm from the floor to present an auditory cue. - Connect the red and black clips of the shock intensity calibrator (i.e., positive and negative connectors) to two any different rods on the grid floor. Connect the USB cable to the corresponding port of the computer. Make sure to connect the red and black clips to bars separated by another bar.
NOTE: This section describes the shock intensity calibration process using a specific brand of equipment mentioned in the Table of Materials. However, the calibration process can be performed using different brands of equipment. It is recommended to calibrate the intensity of the shock in three sectors of the grid floor to verify that it is consistent. In addition, always remove fecal and urine residues from the grid floor to avoid interference during the delivery of the shock. - Start the shock-intensity calibrator software (Table of Materials). Choose an intensity of 1.0 mA in the application by clicking on the range arrow. Then, change the Run/Stop switch to Run.
NOTE: We propose 1.0 mA based on our studies with rodent models in our lab and literature that reports a range from 0.75 mA to 1.5 mA as adequate for studies of fear conditioning33,34,35. - Switch on the aversive stimulator or the equipment used to deliver the footshocks and look at the shock intensity displayed on the panel of the application. If needed, adjust the intensity to 1.0 mA using the knob on the aversive stimulator.
NOTE: Aversive stimulator should be set to “OUT” to appropriately test, calibrate, and run the experiment.
3. Freezing detection system calibration
- Close the experimental chamber and sound-attenuating box doors. Do not introduce the animal at this point, as it will be placed into the chamber after the freezing detection system calibration has been completed. Check that the light intensity inside the box is between 20 and 30 lux.
- Start the freezing detection system software and open the Experiment setup dialogue window. Enter the details of each subject (such as subject identification number, date and group) and load the file titled “Training protocol VFC.pro” (available at http://doi.org/10.17605/OSF.IO/4NKFQ).
NOTE: Context and cue tests use a different program configuration; thus, make sure to use the correct file on each test. At this point the correct file corresponds to “Training protocol VFC.pro”. Remember that during test phases the file corresponding will be different to training session. - Choose the corresponding camera(s) and check the Save Video option (if needed). Set the Motion Threshold to 100, and Min Freeze Duration to 30 frames.
NOTE: This Motion Threshold value is based on the size of the species used (based on number of pixels). Minimum Freeze Duration value is recommended by the manufacturer. These values are used to ensure proper recognition of the animal in the chamber. - Verify that the live feed from the chosen camera(s) appears on the screen, together with the motion threshold graph and the timeline of the different stimuli that are presented during the training (e.g., sound and shock).
NOTE: Using a different brand, the equipment setup should offer the possibility to measure the movements of the animal to detect an “index” of motion that should allow comparisons on the amount of time the animal is moving or freezing. Another possibility is using a software that with only the video source (during or after the experiment) can detect the amount of time the animal is in motion or freezing, such as free software ImageFZ13, open-source toolbox in Matlab14, or a free classifier of animal behavior as JAABA15. - Click the Calibrate option three times, while checking that the Motion Index remains below 100 (threshold). Then, set the equipment to lock by clicking on the corresponding button on the screen.
NOTE: This section describes a freezing detection system calibration process using a specific brand of equipment listed in the Table of Materials. As was mentioned before, the calibration process can be conducted using different brands of equipment (for a review of different options in equipment and software see Anagnostaras et al. 2010)16.
4. Extended fear conditioning training
- Transport the rats in their home cages, covered with a cloth, from the animal care facility to the behavioral training room in the laboratory. Avoid exposure to noise or stress-generating conditions during the transport of animals to the behavioral training room. If several animals are transported at the same time, only bring the animals to be tested and maintain other rats in a holding room to enhance experimental control. Gently handle the animals for 2 min before starting the training.
NOTE: In the protocol, the animals were handled each day for 2 minutes before behavioral training. Following handling, animals were introduced in the experimental chamber. We recommended to manipulate animals to make rats habituate to the researcher. - Introduce the rat to the experimental chamber. Handle it gently by the base of its tail and place it on the middle of the chamber. Close the experimental chamber and sound-attenuating box doors.
- Start the session by clicking on the Record button. Let the rat acclimate to the chamber for 3 min. This 3 min period is the standard recommended by the equipment manufacturer and serves as a baseline and habituation time to the chamber.
- Deliver twenty-five tone-shock pairings (trials) with a 60 s Inter-Trial Interval (ITI), starting on minute 3 of the session. Present the tone (conditioned stimulus – CS; 90 dB SPL, 2000 Hz, 50-ms Rise Time) during the last 10 s of each ITI, and the shock (unconditioned stimulus – US) during the last 2 s of each ITI.
NOTE: Activation of the Record button is conditional on cameras being properly calibrated and locked. - Remove the rat from the experimental chamber when the 28 min session is over. Return animals to the respective home cage. Transport the rats in their home cages covered with a cloth from the behavioral training room to the animal care facility.
- Repeat freezing detection system calibration (steps 3.1-3.5) and fear conditioning (steps 4.1 and 4.3) to train all the subjects.
NOTE: We strongly recommend recalibrating the detection system for each animal to ensure that the software maintains the same parameters when it processes information for freezing detection. - Resting period: During this period, have the animals rest individually in their home cages. Monitor the weight of the animals twice per week during the 6 weeks of the incubation period. Gently manipulate each animal for two min while they are weighted.
5. Context test – single 10 min session
- After the training phase, expose the animals to the first memory test called context test. During this 10 min phase, expose the rats to the same context in which training took place but do not present cues or shocks. Transport the rats in their covered home cages (e.g., with a cloth) from the animal care facility to the behavioral training room. Keep in mind that animals were divided into groups, thus one group is tested 48 h after the training phase and the other group is tested 6 weeks after training (see Figure 1).
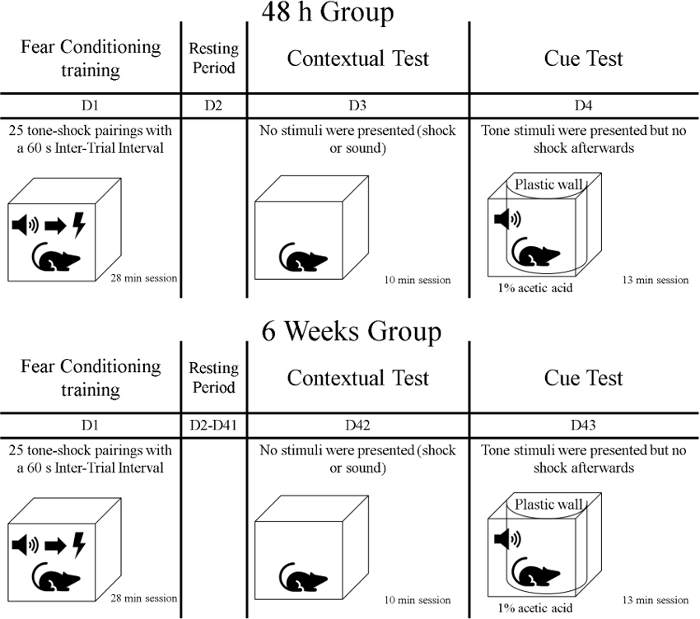
Figure 1: Timeline of the experiment. Please click here to view a larger version of this figure.
- Clean all the internal surfaces of the experimental chamber and stainless-steel grid floor with 10% ethanol. Repeat before testing each animal.
- Repeat freezing detection system calibration (steps 3.1 to 3.5). Open the Experiment setup dialogue window and load the file named “Context test protocol.pro”, which is available from http://doi.org/10.17605/OSF.IO/4NKFQ.
NOTE: This file contains the setup for this experimental phase that consists of no shocks or tones. - Introduce the animal to the experimental chamber. Handle it gently by the base of its tail and place it on the middle of the chamber. Close the experimental chamber and sound-attenuating box doors.
- Start the session by clicking on the Record button. During this single 10 min context-test session, no stimuli are presented (shock neither sound).
- Remove the subject from the experimental chamber when the 10 min session is over. Return the animals to their respective cages and transport the rats in their covered home cages from the behavioral training room to the animal care facility. Repeat steps 5.2-5.5 to test all the subjects.
6. Cue test – single 13 min session
- One day after the context test, have animals undergo the second test of memory called cue test. During this phase, the rats will be in a different context of training during 13 min.; cues (tones) are presented, but no shocks are delivered. Transport the rats in their home cages covered with a cover from the animal care facility to the behavioral training room. Test a group 72 h after the fear conditioning training, and test another group 6 weeks and one day after training (Figure 1).
NOTE: A different system of transportation (from the animal care facility to the experimental room) could be implemented to differentiate more the context and cue tests. Since the animals were transported to the training session and context test session in their home cages, a different transport cage, bedding and/or cover could be used during transportation to the cue test session. - Clean all the internal surfaces of the experimental chamber and stainless-steel grid floor with 10% ethanol. Repeat before testing each animal.
- To change the visual context, insert the plastic surrounding wall to the experimental chamber.
- To change olfactory context, apply 1% acetic acid to a cotton-tipped swab, and place it in the metal tray below the grid floor17,18,19.
- Repeat the freezing detection system calibration (steps 3.1-3.5). Load the file named file “Cue test protocol.pro” file, which is available from http://doi.org/10.17605/OSF.IO/4NKFQ.
NOTE: This file contains the setup for this experimental phase, which consists of delivery of the same tones presented during the training phase (CS), but in the absence of shocks (US). - Introduce the animal to the experimental chamber. Handle it gently by the base of its tail and place it on the middle of the chamber. Close the experimental chamber and sound-attenuating box doors.
- Start the session by clicking on the Record button. During the single 13 min cue test session, the CS stimulus (tone) is presented 10 times, starting on minute 3 of the session.
NOTE: The first 3 min correspond to the baseline of this session, followed by 10 cue test trials (that is, 10 s each) delivered with 50 s ITIs in the absence of shocks. The delivery of tones is automatic, via using the previously loaded file. - Remove the animal from the experimental chamber when the 13 min session is over. Return animals to the respective cage and transport them covered to the animal care facility. Repeat steps 6.2 through 6.5 to test all the subjects.
7. Data analysis
- Obtain the general activity index (i.e., motion index) that is derived from the video stream using the freezing detection system software. This software automatically transforms the motion index to provide the percentage of freezing time per session and the number of freezing episodes. Set the freezing threshold to the default Minimum Freeze Duration setting of the system (1 s = 30 frames).
- Use the additional custom-made program (file available from http://doi.org/10.17605/OSF.IO/4NKFQ) to obtain:
- Use the program to determine the percentage of freezing during the first three minutes of the training session (i.e., baseline freezing, since no shocks or tones were presented before or during that 3 min period) and during the first three minutes of the cue test session.
- Use the program to determine the percentage of freezing for each of eight 3 min bins of the training session.
- Use the program to determine the percentage of freezing during the cue presentations (i.e., freezing during the tones) and no-cue periods (intertrial intervals; ITIs), for both training and cue-test sessions.
- To obtain these data, open the freezing detection system software.
- Select File | Reports | Batch Component summary.
- Select the file with extension .CMP available from http://doi.org/10.17605/OSF.IO/4NKFQ.
- Name the output file and change Motion Threshold to a 100. Then, click OK.
- Select the files to be analyzed (extension .RAW). These files are automatically generated from the freezing detection system software when the session is over and correspond to the raw data of each session. Initially, the files are saved in the desktop of the computer, but they can be stored in a custom folder (e.g., Documents-Fear conditioning) to facilitate their subsequent identification and opening when they need to be analyzed.
- Open the output files (extension .CSV). They can be edited in a spreadsheet software for further analysis. This file contains the results of freezing during the experimental session.
NOTE: To obtain the total percentage of freezing, divide the time that the subject spent immobile over the total session time. The number of freezing episodes can be calculated counting the number of freezing events through the session. In both cases, it is necessary to define a motion threshold based on a minimum freeze duration. This is the temporal criterion that defines whether a Freeze Episode is recorded. Automated systems of recording can use certain amount of frames per second (fps) as a measure of minimum freeze duration. For instance, with a sample rate of 30 fps, a minimum freeze duration of 15 frames will record as freezing an instance of immobility that last for 30 s.
- Calculate the average duration of each freezing episode for each session (training and both tests, context and cue) by dividing the total freezing duration (in seconds) over the total number of freezing episodes.
Access restricted. Please log in or start a trial to view this content.
Wyniki
Variations in percentage of freezing time during different stages of the training session were analyzed for all subjects (n = 12) using a dependent t test (Table 1). Animals were active and explored the experimental chamber during the first three minutes of the training session (first day of the protocol), time during which no tones or shocks were delivered (i.e., baseline-BL). As shown in Figure 2A, percentage of freezing time during th...
Access restricted. Please log in or start a trial to view this content.
Dyskusje
The present extended fear-conditioning protocol is an efficient and valid approach to assess emotional memory across short (48 h) and long-term periods (6 weeks). Thus, the protocol allows to study overtraining and fear incubation phenomena in rats. Among the different advantages of this protocol are the following. It offers two types of memory tests, namely context and cue, that allow to identify the differential effect of two delays (48 h and 6 weeks) across context and cue manipulations. Second, the task entails a sin...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
Financial support for this research was provided by Fundación Universitaria Konrad Lorenz - grant number 9IN15151. The authors would like to thank the Communications Department at Konrad Lorenz University for their help with recording and editing the video, in particular Natalia Rivera and Andrés Serrano (Producers). Also, Nicole Pfaller-Sadovsky and Lucia Medina for their comments on the manuscript, and Johanna Barrero, Dean at Corporacion Universitaria Iberoamericana, for institutional collaboration. The authors have no conflicts of interest.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| Acetic acid (ethanoic acid) | https://pubchem.ncbi.nlm.nih.gov/compound/acetic_acid | ||
| Aversive Stimulation Current Package | MED Associates Inc | ENV-420 | https://www.med-associates.com/product-category/video-fear-packages-for-rat/ |
| Contextual test protocol.pro | http://doi.org/10.17605/OSF.IO/4NKFQ | ||
| Cue test protocol.pro | http://doi.org/10.17605/OSF.IO/4NKFQ | ||
| Curved Wall Insert | MED Associates Inc | VFC-008-CWI | https://www.med-associates.com/product-category/video-fear-packages-for-rat/ |
| Data processing.zip | http://doi.org/10.17605/OSF.IO/4NKFQ | ||
| NIR/White Light Control Box | MED Associates Inc | NIR-100 | |
| Quick Change Floor/Pan Unit for Mouse | MED Associates Inc | ENV-005FPU-M | https://www.med-associates.com/product-category/video-fear-packages-for-rat/ |
| Small Tabletop Cabinet and Power Supply | MED Associates Inc | SG-6080D | https://www.med-associates.com/product-category/video-fear-packages-for-rat/ |
| Standalone Aversive Stimulator/Scrambler (115 V / 60 Hz) | MED Associates Inc | ENV-414S | https://www.med-associates.com/product-category/video-fear-packages-for-rat/ |
| Standard Fear Conditioning Chamber | MED Associates Inc | VFC-008 | https://www.med-associates.com/product-category/video-fear-packages-for-rat/ |
| Training protocol VFC.pro | http://doi.org/10.17605/OSF.IO/4NKFQ | ||
| Video Fear Conditioning Package for Rat | MED Associates Inc | MED-VFC-SCT-R | https://www.med-associates.com/product-category/video-fear-packages-for-rat/ |
Odniesienia
- Frankland, P. W., Bontempi, B. The organization of recent and remote memories. Nature Reviews Neuroscience. 6 (2), 119-130 (2005).
- Suzuki, A., Mukawa, T., Tsukagoshi, A., Frankland, P. W., Kida, S. Activation of LVGCCs and CB1 receptors required for destabilization of reactivated contextual fear memories. Learning & Memory. 15 (6), 426-433 (2008).
- Hermans, E. J., et al. How the amygdala affects emotional memory by altering brain network properties. Neurobiology of Learning and Memory. 112, 2-16 (2014).
- Moryś, J., Berdel, B., Jagalska-Majewska, H., ŁUczyńSka, A. The basolateral amygdaloid complex -its development, morphology and functions. Folia Morphologica. 58 (3), 29-46 (1998).
- LeDoux, J. E. Emotional memory systems in the brain. Behavioural Brain Research. 58 (1-2), 69-79 (1993).
- Labar, K. S. Beyond fear: Emotional memory mechanisms in the human brain. Current Directions in Psychological Science. 16 (4), 173-177 (2007).
- Izquierdo, I., Furini, C. R. G., Myskiw, J. C. Fear Memory. Physiological Reviews. 96 (2), 695-750 (2016).
- Maren, S. Overtraining Does Not Mitigate Contextual Fear Conditioning Deficits Produced by Neurotoxic Lesions of the Basolateral Amygdala. The Journal of Neuroscience. 18 (8), 3097-3097 (1998).
- Pickens, C. L., Golden, S. A., Nair, S. G. Incubation of fear. Current Protocols in Neuroscience. 64, Unit-6.27 (2013).
- Morrow, J. D., Saunders, B. T., Maren, S., Robinson, T. E. Sign-tracking to an appetitive cue predicts incubation of conditioned fear in rats. Behavioural Brain Research. 276, 59-66 (2015).
- Pickens, C. L., Golden, S. A., Adams-Deutsch, T., Nair, S. G., Shaham, Y. Long-lasting incubation of conditioned fear in rats. Biological Psychiatry. 65 (10), 881-886 (2009).
- Schaap, M. W. H., et al. Nociception and Conditioned Fear in Rats: Strains Matter. PLoS ONE. 8 (12), 83339(2013).
- Shoji, H., Takao, K., Hattori, S., Miyakawa, T. Contextual and Cued Fear Conditioning Test Using a Video Analyzing System in Mice. Journal of Visualized Experiments. (85), e50871(2014).
- Patel, T. P., et al. An open-source toolbox for automated phenotyping of mice in behavioral tasks. Frontiers in Behavioral Neuroscience. 8, 349(2014).
- Kabra, M., Robie, A. A., Rivera-Alba, M., Branson, S., Branson, K. JAABA: Interactive machine learning for automatic annotation of animal behavior. Nature Methods. 10 (1), 64-67 (2013).
- Anagnostaras, S. G. Automated assessment of Pavlovian conditioned freezing and shock reactivity in mice using the VideoFreeze system. Frontiers in Behavioral Neuroscience. 4 (58), (2010).
- Moyer, J. R., Brown, T. H. Impaired Trace and Contextual Fear Conditioning in Aged Rats. Behavioral Neuroscience. 120 (3), 612-624 (2006).
- Schuette, S. R., Hobson, S. Conditioned contextual fear memory to assess natural forgetting and cognitive enhancement in rats. Journal of Biological Methods. 5 (3), 99(2018).
- Chang, C. H., et al. Fear extinction in rodents. Current Protocols in Neuroscience. , Chapter 8 (SUPPL. 47) (2009).
- Pickens, C. L., Golden, S. A., Nair, S. G. Incubation of fear. Current Protocols in Neuroscience. 64, 1-18 (2013).
- Izquierdo, I., Furini, C. R. G., Myskiw, J. C. Fear Memory. Physiological Reviews. 96 (2), 695-750 (2016).
- Vetere, G., et al. Chemogenetic Interrogation of a Brain-wide Fear Memory Network in Mice Article Chemogenetic Interrogation of a Brain-wide Fear Memory Network in Mice. Neuron. 94 (2), 363-374 (2017).
- Koob, G. F., Zimmer, A. Chapter 9 - Animal models of psychiatric disorders. Neurobiology of Psychiatric Disorders. 106, 137-166 (2012).
- Bourin, M. Animal models for screening anxiolytic-like drugs: a perspective. Dialogues in clinical neuroscience. 17 (3), 295-303 (2015).
- Murray, S. B., et al. Fear as a translational mechanism in the psychopathology of anorexia nervosa. Neuroscience & Biobehavioral Reviews. 95, 383-395 (2018).
- Pamplona, F. A., et al. Prolonged fear incubation leads to generalized avoidance behavior in mice. Journal of Psychiatric Research. 45 (3), 354-360 (2011).
- Török, B., Sipos, E., Pivac, N., Zelena, D. Modelling posttraumatic stress disorders in animals. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 90, 117-133 (2019).
- Bhakta, A., Gavini, K., Yang, E., Lyman-Henley, L., Parameshwaran, K. Chronic traumatic stress impairs memory in mice: Potential roles of acetylcholine, neuroinflammation and corticotropin releasing factor expression in the hippocampus. Behavioural Brain Research. 335, 32-40 (2017).
- Uniyal, A., et al. Pharmacological rewriting of fear memories: A beacon for post-traumatic stress disorder. European Journal of Pharmacology. , 172824(2019).
- Barad, M. Fear extinction in rodents: basic insight to clinical promise. Current Opinion in Neurobiology. 15 (6), 710-715 (2005).
- Haaker, J., et al. Making translation work: Harmonizing cross-species methodology in the behavioural neuroscience of Pavlovian fear conditioning. Neuroscience & Biobehavioral Reviews. 107, 329-345 (2019).
- Heroux, N. A., Horgan, C. J., Pinizzotto, C. C., Rosen, J. B., Stanton, M. E. Medial prefrontal and ventral hippocampal contributions to incidental context learning and memory in adolescent rats. Neurobiology of Learning and Memory. 166, 107091(2019).
- Rossi, M. A., Yin, H. H. Methods for Studying Habitual Behavior in Mice. Current Protocols in Neuroscience. 60 (1), 8-29 (2012).
- Brady, A. M., Floresco, S. B. Operant Procedures for Assessing Behavioral Flexibility in Rats. Journal of Visualized Experiments. (96), (2015).
- Zoccolan, D., Di Filippo, A. Methodological Approaches to the Behavioural Investigation of Visual Perception in Rodents. Handbook of Behavioral Neuroscience. , Elsevier B.V. (2018).
- Lguensat, A., Bentefour, Y., Bennis, M., Ba-M'hamed, S., Garcia, R. Susceptibility and Resilience to PTSD-Like Symptoms in Mice Are Associated with Opposite Dendritic Changes in the Prelimbic and Infralimbic Cortices Following Trauma. Neuroscience. 418, 166-176 (2019).
- Li, Q., et al. N-Acetyl Serotonin Protects Neural Progenitor Cells Against Oxidative Stress-Induced Apoptosis and Improves Neurogenesis in Adult Mouse Hippocampus Following Traumatic Brain Injury. Journal of Molecular Neuroscience. 67 (4), 574-588 (2019).
- Pantoni, M. M., Carmack, S. A., Hammam, L., Anagnostaras, S. G. Dopamine and norepinephrine transporter inhibition for long-term fear memory enhancement. Behavioural Brain Research. 378 (112266), 112266(2020).
- Smith, K. L., et al. Microglial cell hyper-ramification and neuronal dendritic spine loss in the hippocampus and medial prefrontal cortex in a mouse model of PTSD. Brain, Behavior, and Immunity. 80, 889-899 (2019).
- Liu, X., Zheng, X., Liu, Y., Du, X., Chen, Z. Effects of adaptation to handling on the circadian rhythmicity of blood solutes in Mongolian gerbils. Animal Models and Experimental. 2 (2), 127-131 (2019).
- Landgraf, D., McCarthy, M. J., Welsh, D. K. The role of the circadian clock in animal models of mood disorders. Behavioral Neuroscience. 128 (3), 344-359 (2014).
- Refinetti, R., Kenagy, G. J. Diurnally active rodents for laboratory research. Laboratory annimals. 52 (6), 577-587 (2018).
- Hurtado-Parrado, C., et al. Assessing Mongolian gerbil emotional behavior: effects of two shock intensities and response-independent shocks during an extended inhibitory-avoidance task. PeerJ. 5, (2017).
- Frey, P., Eng, S., Gavinf, W. Conditioned suppression in the gerbil. Behavior Research Methods & Instrumentation. 4 (5), 245-249 (1972).
- Woolley, M. L., Haman, M., Higgins, G. A., Ballard, T. M. Investigating the effect of bilateral amygdala lesions on fear conditioning and social interaction in the male Mongolian gerbil. Brain Research. 1078 (1), 151-158 (2006).
- Ballard, T. M., Sänger, S., Higgins, G. a Inhibition of shock-induced foot tapping behaviour in the gerbil by a tachykinin NK1 receptor antagonist. European Journal of Pharmacology. 412 (3), 255-264 (2001).
- Luyten, L., Schroyens, N., Hermans, D., Beckers, T. Parameter optimization for automated behavior assessment: plug-and-play or trial-and-error. Frontiers in Behavioral Neuroscience. 8 (28), (2014).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone