Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Systems Analysis of the Neuroinflammatory and Hemodynamic Response to Traumatic Brain Injury
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
This protocol presents methods to characterize the neuroinflammatory and hemodynamic response to mild traumatic brain injury and to integrate these data as part of a multivariate systems analysis using partial least squares regression.
Streszczenie
Mild traumatic brain injuries (mTBIs) are a significant public health problem. Repeated exposure to mTBI can lead to cumulative, long-lasting functional deficits. Numerous studies by our group and others have shown that mTBI stimulates cytokine expression and activates microglia, decreases cerebral blood flow and metabolism, and impairs cerebrovascular reactivity. Moreover, several works have reported an association between derangements in these neuroinflammatory and hemodynamic markers and cognitive impairments. Herein we detail methods to characterize the neuroinflammatory and hemodynamic tissue response to mTBI in mice. Specifically, we describe how to perform a weight-drop model of mTBI, how to longitudinally measure cerebral blood flow using a non-invasive optical technique called diffuse correlation spectroscopy, and how to perform a Luminex multiplexed immunoassay on brain tissue samples to quantify cytokines and immunomodulatory phospho-proteins (e.g., within the MAPK and NFκB pathways) that respond to and regulate activity of microglia and other neural immune cells. Finally, we detail how to integrate these data using a multivariate systems analysis approach to understand the relationships between all of these variables. Understanding the relationships between these physiologic and molecular variables will ultimately enable us to identify mechanisms responsible for mTBI.
Wprowadzenie
Overview
Mild traumatic brain injuries (mTBIs) impact ~1.6-3.8 million athletes annually1. These injuries, including sub-concussive and concussive injuries, can leave patients with transient physical, emotional, psychological and cognitive symptoms2. Moreover, repetitive mTBI (rmTBI) sustained within a “window of vulnerability” can lead to cumulative severity and duration of cognitive consequences that last longer than the effects of a single mTBI alone3, and ultimately even to permanent loss of function4,5,6. Although many patients recover within a relatively short time frame (<1 week), 10-40% of patients suffer longer lasting effects of mTBI for > 1 month, with some lasting up to 1 year3,7,8,9. Despite the high prevalence and lasting consequences of these injuries, injury mechanisms are poorly understood and no effective treatment strategies exist.
Given the high variability in outcomes after mTBI/rmTBI, one challenge in identifying early-stage molecular triggers from tissue obtained in terminal mTBI/rmTBI studies is the lack of longitudinal data demonstrating definitive "acute molecular links" of these molecular triggers to longer-term outcomes. To overcome this challenge, our group has discovered that acutely reduced cerebral blood flow measured acutely using an optical tool called diffuse correlation spectroscopy (DCS), strongly correlates with longer-term cognitive outcome in a mouse model of rmTBI10. Using this hemodynamic biomarker, we showed that mice with acutely low cerebral blood flow (and, by extension, worse predicted long-term outcome) have concomitant acute increases in neuronal phospho-signaling within both MAPK and NFκB pathways, increases in neuronal expression of pro-inflammatory cytokines, and increases in expression of the phagocyte/microglial marker Iba111. These data suggest a possible role for neuronal phospho-signaling, cytokine expression, and microglial activation in both the acute regulation of cerebral blood flow post injury as well as in triggering a signaling cascade that leads to neuronal dysfunction and worse cognitive outcome. Herein, we detail our approach to simultaneously probe both the hemodynamic and neuroinflammatory environment after rmTBI and how to integrate these complex datasets. Specifically, we outline procedures for four key steps to this comprehensive approach: (1) a weight-drop model of mild traumatic brain injury, (2) assessment of cerebral blood flow with diffuse correlation spectroscopy, (3) quantification of the neuroinflammatory environment, and (4) data integration (Figure 1). Below, we provide a brief introduction to each of these key steps to help guide readers through the rationale behind our methods. The remainder of the manuscript provides a detailed protocol for each of these key steps.
Weight-drop model of mild traumatic brain injury
Although many excellent preclinical models of repetitive mild TBI exist12,13,14,15,16,17,18, we employ a well-established and clinically relevant weight-drop closed head injury model. Key features of this model include (1) blunt impact of the intact skull/scalp followed by unrestricted rotation of the head about the neck, (2) no overt structural brain injury, edema, blood–brain barrier damage, acute cell death, or chronic brain tissue loss, and (3) persistent (up to 1 year) cognitive deficits that emerge only after multiple hits19 (Figure 2).
Assessment of cerebral blood flow with diffuse correlation spectroscopy
Diffuse correlation spectroscopy (DCS) is a non-invasive optical technique that measures blood flow5,20,21. In DCS, a near-infrared light source is placed on the tissue surface. A detector is placed at a fixed distance from the source on the tissue surface to detect light that has multiply scattered through the tissue (Figure 3). Scattering off moving red blood cells causes the detected light intensity to fluctuate with time. A simple analytical model known as correlation diffusion theory is used to relate these intensity fluctuations to an index of blood flow (CBFi, Figure 4). Although the units of CBFi (cm2/s) are not the traditional units of flow (mL/min/100 g), a previous study in mice has shown that CBFi strongly correlates with cerebral blood flow measured by arterial spin labeled MRI21.
For reference, the DCS instrument used here was built in-house and is comprised of an 852 nm long coherence-length laser, an array of 4 photon counting avalanche photodiodes, and a hardware autocorrelator board (single tau, 8 channel, 100 ns minimum sample time)21,22. Data is acquired with homemade software written in LabView. The animal interface for the device consists of a 400 μm multimode source fiber (400-2200 nm wavelength range, pure silica core, TECS Hard Cladding) and a 780 nm single mode detector fiber (780-970 nm wavelength range, pure silica core, TECS Hard Cladding, 730 ± 30 nm second mode cut-off) spaced 6 mm apart and embedded in a black 3D-printed sensor (4 mm x 8 mm, Figure 3).
Quantification of the neuroinflammatory environment
Although neuroinflammation is regulated by diverse cellular processes, two key relevant mechanisms are extracellular signaling by cytokines/chemokines and intracellular signaling by phospho-proteins. To investigate the neuroinflammatory environment of the brain post-injury, brains are extracted from mice, microdissected, and cytokines/chemokines and phospho-proteins are quantified using Luminex (Figure 5, Figure 6, Figure 7). Luminex multiplexed immunoassays enable simultaneous quantification of a diverse collection of these proteins by coupling enzyme-linked immunosorbent assays (ELISAs) to fluorescently tagged magnetic beads. Distinct fluorescent tags are used for each protein of interest, and beads of each tag are functionalized with a capture antibody against that particular protein. Hundreds of beads for capturing each protein are mixed together, placed in a 96 well plate, and incubated with sample. After sample incubation, a magnet is used to trap the beads in the well while the sample is washed out. Next, biotinylated detection antibody binds to the analyte of interest to form an antibody-antigen sandwich similar to a traditional ELISA, but with the ELISA for each protein occurring on a different fluorescently tagged bead. Adding phycoerythrin-conjugated streptavidin (SAPE) completes each reaction. The Luminex instrument then reads the beads and separates the signal according to each fluorescent tag/protein.
Data integration
Because of the large number of analytes (e.g., cytokines) measured in the Luminex assay, data analysis can be difficult to interpret if each quantified protein is analyzed individually. To simplify analysis and to capture trends observed among analytes, we use a multivariate analysis method called partial least squares regression (PLSR, Figure 8)23. PLSR works by identifying an axis of weights corresponding to each measured protein (i.e., cytokines or phospho-proteins, referred to as “predictor variables”) that together optimally explain co-variance of the measured proteins with a response variable (e.g., cerebral blood flow). The weights are referred to as “loadings” and are assembled into a vector known as a latent variable (LV). By projecting (referred to as “scoring”) the measured protein data on each of two LVs, the data can be re-plotted in terms of these LVs. After computing the PLSR, we use a varimax rotation to identify a new LV that maximizes the covariance between the sample projections onto the LV and the predictor variable24. This approach allows us to define LV1 as the axis for which the variance of the response variable is best explained. LV2 maximizes co-variance between the response variable and LV1 residual data, which may be associated with biological or technical variability between samples. Lastly, we conduct a Leave One Out Cross Validation (LOOCV) to ensure that the PLSR model is not heavily dependent upon any one sample23.
In this protocol, we detail methods to characterize the neuroinflammatory and hemodynamic tissue response to mTBI. The general workflow is outlined in Figure 1. In this protocol, mice are subject to one or more mTBIs using a weight-drop closed head injury model. Cerebral blood flow is measured longitudinally before and at multiple time points after injury. At the time point of interest for interrogation of neuroinflammatory changes, the animal is euthanized, and the brain is extracted. Brain regions of interest are isolated via microdissection and then lysed to extract protein. Lysates are then used for both Luminex multiplexed immunoassays of cytokine and phospho-protein expression as well as Western blot. Finally, this holistic dataset is integrated using a partial least squares regression analysis.
Protokół
All animal procedures are approved by Emory University Institutional Animal Care and Use Committee (IACUC) and followed the NIH Guidelines for the Care and Use of Laboratory Animals.
1. Weight-drop model of mild traumatic brain injury
- Prepare the weight-drop setup. Mount a vise on a flat surface with a 1 m guide tube (2.54 cm inner diameter) aligned vertically (check using a level). Use a 54 g bolt (0.95 cm basic body diameter, 2 cm head diameter, 10.2 cm length) for the impact.
- Briefly anesthetize mouse. Induce the mouse with 4.5% isoflurane in 100% oxygen for 45 seconds. Confirm sufficient depth of anesthesia by the lack of a toe pinch response.
- Induce injury.
- Rapidly remove the mouse from anesthesia and place the mouse prone on the center of a thin membrane (11.2 cm x 21.3 cm tissue).
- Use both hands to hold the tissue taut with the mouse prone on the center. Secure the mouse’s tail under a thumb. Position the mouse head under the guide tube (Figure 2).
- Drop the bolt from the top of the guide tube onto the dorsal aspect of the mouse’s head, aiming for impact between the back of the eyes and the front of the ears. Upon impact, the mouse will penetrate the tissue, allowing for rapid acceleration of the head about the neck (Figure 2).
- Recovery
- After impact, place the mouse supine on a 37 °C warming pad in room air. Monitor recovery for 1 h post-injury. Within 1 h, mice should be able to ambulate normally, find food and water, and not exhibit gross motor deficits.
NOTE: Analgesia is not used per approval of the Institutional Animal Care and Use Committee, which is justified due to the confounding influence of analgesia on the parameters of interest (i.e., cerebral blood flow, markers of inflammation). Loss of consciousness, defined as the time from removal from anesthesia to the time to regain righting reflex, is expected and typically lasts from 20 s to 3 minutes (Supplemental Table 1). Brief (<30 s) episodes of apnea and/or seizure-like activity may be observed, particularly after repetitive head injuries spaced once-daily.
- After impact, place the mouse supine on a 37 °C warming pad in room air. Monitor recovery for 1 h post-injury. Within 1 h, mice should be able to ambulate normally, find food and water, and not exhibit gross motor deficits.
- Repeat as needed. This injury may be repeated once-daily, weekly, or monthly. The number and spacing of injuries depend on desired injury severity. Typically, we employ five hits spaced once daily to induce robust deficits in spatial learning and memory.
NOTE: Prior studies have shown that five hits spaced once-daily is sufficient to induce deficits in spatial learning and memory lasting over 1 year post-injury without edema, hemorrhage, or overt structural injury to the brain19. Mice are weighed daily and closely monitored for signs of dehydration, motor deficits, and loss of appetite. If dehydrated, mice are given moist chow and a subcutaneous injection of 1 mL of saline once daily. In order to prevent unnecessary suffering and to ensure a humane endpoint, mice are euthanized if: dehydration persists or worsens >24 h post-subcutaneous saline treatment, body weight decreases by more than 20% from pre-injury baseline, motor deficits such as circling or paw-dragging appear and persist >1 h after injury.
2. Assessment of cerebral blood flow with diffuse correlation spectroscopy
- DCS data acquisition
- Remove hair on the scalp. Because DCS works best in the absence of hair, it is necessary to remove fur on the head prior to the start of experiments. Typically, hair removal is done 1-3 days prior to the start of the study.
- Induce mice with 4.5% isoflurane in 100% oxygen for 45 seconds and maintain with 1-2% isoflurane in 100% oxygen.
- Shave the head between the eyes and the ears. Then, use depilatory cream to remove fur on the head as in Figure 3.
- Allow the animal to recover from anesthesia on a warming pad and then return to cage.
- Measure cerebral blood flow with DCS. To minimize motion artifacts during measurement, study mice under brief isoflurane anesthesia.
NOTE: Visually monitor respiration and toe pinch response throughout measurements and adjust isoflurane concentration as needed to ensure consistent depth of anesthesia. Significant variations in the depth of anesthesia could alter blood flow given the known vasomodulatory effects of isoflurane25.- Induce with 4.5% isoflurane in 100% oxygen for 45 seconds, and then maintain with 1.0-1.75% isoflurane in 100% oxygen. Confirm sufficient depth of anesthesia by the absence of a toe pinch response and normal respiration (between ~60-80 breaths per minute).
- After a 2 min period of stabilization, gently rest the DCS sensor over the right hemisphere such that the top edge of the optical sensor lines up with the back of the eye and the side of the sensor lines up along the midline (Figure 3). Cup a hand over the sensor to shield from room light. Acquire 5 seconds of data (1 Hz acquisition).
- Reposition the sensor over the left hemisphere, and acquire 5 seconds of data.
- Repeat 3 times/hemisphere to account for local heterogeneities under the tissue surface.
- Recovery
- Remove the mouse from anesthesia and place on a warming pad.
- After the mouse regains its righting reflex return it to the cage.
- Remove hair on the scalp. Because DCS works best in the absence of hair, it is necessary to remove fur on the head prior to the start of experiments. Typically, hair removal is done 1-3 days prior to the start of the study.
- DCS data analysis
- Perform initial quality control. Each frame of DCS data consists of a measured normalized intensity autocorrelation function,
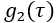 (Figure 4A), and photon count rate (kHz).
(Figure 4A), and photon count rate (kHz).
- To remove data frames with significant motion artifact, discard data frames for which the mean value of the tail of the
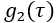 curve (i.e.,
curve (i.e., 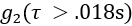 ) is > 1.005.
) is > 1.005. - To remove data frames with poor signal-to-noise ratio, discard data frames if the detected photon count rate is < 20 kHz.
- To remove data frames with significant motion artifact, discard data frames for which the mean value of the tail of the
- Extract cerebral blood flow index. Using fminsearch in Matlab, fit each ith measured data frame
 for CBFi(i). Restrict fits to
for CBFi(i). Restrict fits to 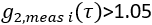 , and find the value of CBFi that minimizes the following cost function:
, and find the value of CBFi that minimizes the following cost function:
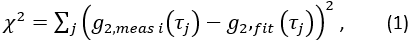
Where the sum is over all measured delay times,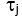 , and
, and 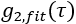 is the semi-infinite homogeneous solution of the correlation diffusion equation (Figure 4B):
is the semi-infinite homogeneous solution of the correlation diffusion equation (Figure 4B):
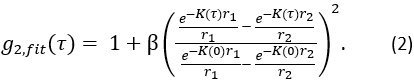
Here β is a coherence factor determined by the experimental set up,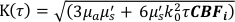 ,
, 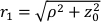 ,
, 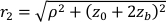 ,
, 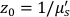 ,
, 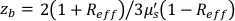 , Reff =0.493 for an assumed tissue index of refraction of 1.4, ρ is 6 mm, and μa and μ's are the absorption and reduced scattering coefficient of the tissue (assumed to be 0.25 and 9.4/cm, respectively10,26,27).
, Reff =0.493 for an assumed tissue index of refraction of 1.4, ρ is 6 mm, and μa and μ's are the absorption and reduced scattering coefficient of the tissue (assumed to be 0.25 and 9.4/cm, respectively10,26,27).
NOTE: Because β can vary ~10% over time, fit each data frame for β and CBFi simultaneously. - Perform secondary quality control. Within each repetition (which consists of 5 data frames), discard outliers. Outliers are defined as those CBFi values that fall outside 1.5 standard deviations of the mean CBFi for that repetition. If more than 1 data point is identified as an outlier, discard the entire repetition.
- Estimate average cerebral blood flow index: Estimate an average CBFi per hemisphere by taking the mean across all data frames for all repetitions (Figure 4C). If no significant hemispheric differences are observed, average across hemispheres to obtain an estimate of average global CBFi.
- Perform initial quality control. Each frame of DCS data consists of a measured normalized intensity autocorrelation function,
3. Multiplexed quantification of cytokines and phospho-proteins using luminex assays
- Tissue extraction
NOTE: Quantification of brain cytokines and phospho-signaling proteins using Luminex requires tissue extraction.- Anesthetize mouse using 4.5% isoflurane in 100% oxygen for 1-2 min. Check for deep plane of anesthesia via the lack of a toe pinch response. Euthanize via decapitation.
- Harvest the tissue.
- Remove the brain. Typically, fix the left hemisphere for histology and microdissect several regions from the right hemisphere within the cortex and hippocampus (Figure 5).
- Place dissected samples in microcentrifuge tubes, flash freeze in liquid nitrogen. For analysis of freeze-sensitive proteins, it is optimal to sub-divide tissue sections prior to flash freezing to avoid later freeze-thaw.
NOTE: The protocol can be paused, and tissue samples can be stored at -80 °C until ready to lyse samples. Alternatively, samples can be lysed, and then stored at -80 °C.
- Lyse samples.
- Prepare the lysis buffer by adding protease inhibitor and 2 mM phenylmethylsulfonyl fluoride to the lysis buffer.
- Add 150 µL of the mixed lysis buffer per approximately 3 µg of animal tissue. For reference, mouse visual cortex tissue samples are approximately 3 µg.
- To homogenize the tissue, mechanically triturate the tissue by pipetting up and down ~15-20 times using a 1000 µL pipette. For optimal sample trituration, a homogenizer pestle can be used.
- Place the sample tubes on a rotator for 30 min at 4°C.
- Centrifuge the samples at 4°C for 10 min at approximately 15,000 x g, and collect the supernatant. Samples may be processed immediately or stored at -80°C for further analysis.
NOTE: Sample lysates prepared using this protocol are compatible with Western blot, from which the phagocyte/microglial marker Iba1 and/or the astrocyte activation marker GFAP can be analyzed to complement cytokine and phospho-protein analysis for neuroinflammation studies11.
- Multiplex immuno-assay protocol for cytokines and phospho-proteins
NOTE: Although similar overall, there are some minor differences in the protocols for cytokine and phospho-protein kits. Differences are noted in each step. The steps to prepare samples for the Luminex assay are outlined below.- Preparation of reagents (day 1, same for cytokines and phospho-proteins)
- Allow reagents to warm to room temperature (~30 min).
- Sonicate multiplex magnetic beads bottle for 30 seconds followed by 1 min of vortexing. Ensure multiplex magnetic beads are shielded from light with aluminum foil or use provided light protective bottles.
- Prepare wash buffer by mixing 0.1% Tween20 in 1xPBS or alternatively use the wash buffer provided in the kit.
- Preparation of lysed tissue samples (day 1, same for cytokines and phospho-proteins)
- If previously frozen, remove lysed tissues samples from freezer and allow to thaw on ice (~20 min). Centrifuge samples for 10 min at 9,167 x g to remove precipitate.
- Prepare 25 µL of sample at the optimal protein concentration determined by the linear range analysis (see section 3.3). To normalize total volume for all samples, dilute samples in assay buffer provided in the kit.
- Preparation of 96 well plate (day 1, same for cytokines and phospho-proteins)
- Use the 96 well plate included in the kit or one with a thin bottom (e.g., Brand Tech).
- Add 200 µL of wash buffer (or 1x PBS, 0.1% Tween) into each well and mix on plate shaker for 10 min at 750 rpm.
- Decant wash buffer and tap the plate onto a paper towel to remove residue.
- Immunoassay procedure for Cytokines (day 1)
- Add the following to each well in order.
- Add 25 µL of assay buffer to all wells.
- Add 25 µL of additional assay buffer ONLY to background wells. For every experimental run, have at least two background wells. Background wells have no sample loaded and define the fluorescent intensity read by the instrument without sample.
- Add 25 µL of each diluted sample to corresponding sample wells.
- Add 25 µL of 1x multiplex magnetic beads to all wells (Figure 6). Be sure to vortex beads for 1 min before adding to wells.
- Add 25 µL of assay buffer to all wells.
- Seal plate with plate sealer and cover the plate with aluminum foil. Incubate overnight (12-16 h) at 2-8 °C.
- Add the following to each well in order.
- Immunoassay procedure for cytokines (day 2)
- Place 96 well plate on magnetic separator, making sure that the wells are aligned with the magnets. Let sit for 2 min. Decant well contents while the plate is still attached to the magnetic separator.
- Wash plate 2 times using the following steps.
- Add 200 µL of wash buffer to each well and place on shaker for 2 min at room temperature.
- Place the well plate on the magnetic separator for 2 min at room temperature.
- Decant well contents while the well plate is still attached to magnetic separator.
- Add 25 µL of detection antibody per well (Figure 6). Cover with foil. Incubate for 1 hour on a plate shaker (750 rpm) at room temperature.
- Leave the detection antibody in and add 25 µL of streptavidin-phycoerythrin (SAPE) to each well (Figure 6). Cover with foil. Incubate for 30 min on plate shaker (750 rpm) at room temperature.
- Place the well plate on a magnetic separator and let sit for 2 min. Decant well contents and detach from magnetic separator.
- Wash well plate two times (see step 3.2.5.2).
- Add 75 µL of Luminex Drive Fluid (if using MAGPIX instrument) to each well or assay buffer (if using 200 or FlexMap 3D instrument). Re-suspend beads on plate shaker for 5 min at room temperature.
- Read on Luminex Instrument (MAGPIX, 200, or FlexMap 3D), referring to the user’s manual for proper operation (Figure 6).
- Immunoassay procedure for phospho-proteins (day 1)
- Add the following to each well in order.
- Add 25 µL of assay buffer to all wells.
- Add 25 µL of additional assay buffer ONLY to background wells. For every experimental run, it is recommended to have at least two background wells. Background wells have no sample loaded and define the fluorescent intensity read by the instrument without sample.
- Add 25 µL of each diluted sample to each sample well.
- Add 25 µL of 1x multiplex magnetic beads to all wells (Figure 6).
NOTE: The Luminex assay kit provides multiplex magnetic bead in 20x stock solution. Be sure to vortex 20x stock multiplex magnetic bead solution for 2 min, and then dilute it in assay buffer to 1x solution. Vortex 1x multiplex magnetic bead suspension for 1 min before adding to wells.
- Seal plate with plate sealer and cover the plate with aluminum foil. Incubate overnight (12-16 hours) at 2-8 °C.
- Add 25 µL of assay buffer to all wells.
- Add the following to each well in order.
- Immunoassay procedure for phospho-proteins (day 2)
- Place the well plate on a magnetic separator, making sure that the well plate is fully aligned with the magnetic separator. Let sit for 2 min. Decant well contents while the well plate is still attached to the magnetic separator.
- Wash plate 2 times (see step b in cytokine’s immunoassay procedure day 2).
- Dilute the 20x stock detection antibody to 1x solution in assay buffer. Add 25 µL of 1x detection antibody per well (Figure 6). Cover with foil. Incubate for 1 h on plate shaker (750 rpm) at room temperature.
- Place the 96 well plate on magnetic separator, and let sit for 2 min. Decant well contents, detach from the magnetic separator.
- Dilute 25x stock SAPE in assay buffer to 1x buffer. Add 25 µL of 1x SAPE (Figure 6). Cover with foil and incubate for 15 min on plate shaker (750 rpm) at room temperature.
- Leave the SAPE in wells, and add 25 µL of amplification buffer to each well. Cover with foil.
- Incubate for 15 min on plate shaker (750 rpm) at room temperature.
- Place the well plate on the magnetic separator for 2 min. Decant well contents and detach from the magnetic separator.
- Add 75 µL of Luminex Drive Fluid (if using MAGPIX instrument) or assay buffer (if using 200 or FlexMap 3D instrument). Re-suspend beads on a plate shaker for 5 min at room temperature.
- Read on Luminex instrument (MAGPIX, 200, or FlexMap 3D), referring to the user’s manual for proper operation (Figure 6).
- Preparation of reagents (day 1, same for cytokines and phospho-proteins)
- Linearity of sample dilution curve
- Preparation of samples: Serially dilute test samples with different concentration of total protein. For bulk brain tissues, load serial dilutions from 0-25 µg for cytokines and 0-12 µg for phospho-proteins. Total protein concentration can be measured using bicinchoninic acid (BCA) assay.
- Multiplex immunoassay: Perform the Luminex assay (see section 3.2) on selected samples.
- Data analysis
- Plot fluorescent intensity for each protein vs. amount of protein loaded (Figure 7).
- For each analyte, identify range of total protein loaded for which the relationship between total protein and the fluorescent intensity readout is linear (Figure 7).
- To determine the amount of total protein that should be loaded for the full assay run, identify the linear portion of the curve for each analyte and then select a protein concentration that falls within the linear range for the majority of analytes.
NOTE: Although most proteins share a similar linear range, the linear ranges may not overlap for all proteins. If this is the case, it may be necessary to run each sample multiple times with different amounts of total protein loaded. Alternatively, nonlinear samples may be left out of the analysis. Additionally, some proteins may not have a linear range whatsoever.
4. Partial least squares regression
NOTE: Sample R code and a sample data spreadsheet are provided to carry out the Partial Least Squares Analysis.
- Data Preparation: Format the data as shown in the provided sample data spreadsheet, “MyData”. Include variable names in the row 1, sample names in column A, the response variable in column B, and all predictor variables in columns C+. Fill the last two rows with the background data, and set both sample names to “Background”.
- Partial Least Squares Regression in RStudio
- Install R from www.r-project.org (free, open source).
- Install RStudio Desktop from www.rstudio.com (free, open source license).
- Download the sample R code provided with this publication, “PLSR_Sample_Code.R” and save it to the same folder that contains the data spreadsheet. Open the code file in RStudio.
- In the User Input section, change “dataFileName” to the name of the data spreadsheet.
- Carry out the following steps by highlighting the section of code to run and clicking Run in the top right corner of the script.
- Load necessary R packages, functions, the working directory address, and user input values in RStudio (subsection “Preliminaries”).
- Load the data into RStudio and prepare raw data for processing by subtracting mean background signal from all measurements and z-scoring each analyte (subsection “Read Data and Subtract Background”)(Figure 8A).
- Perform partial least squares regression in RStudio using the plsRglm package v1.2.528 available on the Comprehensive R Archive Network (CRAN). Perform a varimax rotation (stats package v3.6.2)23 in the LV1-LV2 plane to identify a new horizontal axis that best separate samples by the response variable (subsection “PLS”)(Figure 8B).
- Conduct a Leave One Out Cross Validation (LOOCV) in which one sample is iteratively left out of the data and the PLSR model is re-computed. Compute standard deviation for analyte loadings across all LOOCV runs (subsection “LOOCV”).
- Create representative plots: Run the provided sample code as detailed above to create representative plots which automatically export as pdf files to the working directory (the folder containing the data and code files).
- Create a heat map of the processed data as shown in Figure 8A (subsection “PLS”). Color each entry along a spectrum defined by z-score. Sort analytes by the order computed in the latent variable of interest.
- Create a scores plot with LV1 scores plotted along the horizontal axis and LV2 scores plotted along the vertical axis, as shown in Figure 8B (subsection “PLS”). Color each data point according to its response variable measurement to visualize the relationship between each latent variable and the response variable.
- Create a bar plot displaying loadings for each of your predictor variables to visualize how each analyte contributes to the latent variables, as shown in Figure 8C (subsection “LOOCV”).
- Create a plot regressing LV1 scores against your response variable to visualize how well the PLSR model separates the samples, as shown in Figure 8D (subsection “PLS”).
Wyniki
Previously collected data were taken from prior work in which a group of eight C57BL/6 mice were subjected to three closed-head injuries (Figure 2) spaced once daily11. In this work, cerebral blood flow was measured with diffuse correlation spectroscopy 4 h after the last injury (Figure 3, Figure 4). After post-injury CBF assessment, the animals were euthanized, and brain tissue was extracted for quantificati...
Dyskusje
Herein we detail methods for assessment of the hemodynamic and neuroinflammatory response to repetitive mild traumatic brain injury. Further, we have shown how to integrate these data as part of a multivariate systems analysis using partial least squares regression. In the text below we will discuss some of the key steps and limitations associated with the protocol as well as the advantages/disadvantages of the methods over existing methods.
Weight-drop model of mild traumatic brain in...
Ujawnienia
None.
Podziękowania
This project was supported by the National Institutes of Health R21 NS104801 (EMB) and R01 NS115994 (LBW/EB) and Children’s Healthcare of Atlanta Junior Faculty Focused Award (EMB). This work was also supported by the U.S. Department of Defense through the Congressionally Directed Medical Research Programs under Award No. W81XWH-18-1-0669 (LBW/EMB). Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. 1937971. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Materiały
| Name | Company | Catalog Number | Comments |
| Adjustable pipettes | any adjustable pipette | ||
| Aluminum foil | VWR | 89107-726 | |
| Bio-Plex cell lysis kit | C Bio-Rad | 171304012 | |
| BRAND BRANDplates pureGrade Microplates, Nonsterile | BrandTech | 781602 96 | |
| Complete mini protease inhibitor tablet | Sigma-Aldrich | 11836153001 | |
| Depilatory cream | Amazon | Nair | |
| DiH2O | VWR | VWRL0200-1000 | |
| Handheld magnetic separator block for 96 well flat bottom plates | Millipore Sigma Catalogue | 40-285 | |
| Hardware Autocorrelator Board | www.correlator.com | Flex05-8ch | |
| Isoflurane 250 mL | MED-VET INTERNATIONAL | RXISO-250 | |
| Kimwipe (11.2 x 21.3 cm) | VWR | 21905-026 | |
| Laboratory vortex mixer | VWR | 10153-838 | |
| LabView | National Instruments | LabVIEW | |
| Luminex 200, HTS, FLEXMAP 3D, or MAGPIX with xPONENT software | Luminex Corporation | ||
| Luminex Drive Fluid | Luminex | MPXDF-4PK | |
| Luminex sheath fluid | EMD Millipore | SHEATHFLUID | |
| MILLIPLEX MAP Mouse Cytokine/Chemokine Magnetic Bead Panel - Premixed 32 Plex - Immunology Multiplex Assay | Millipore Sigma | MCYTMAG-70K-PX32 | |
| MILLIPLEX MAPK/SAPK Signaling 10-Plex Kit-Cell Signaling Multiplex Assay | Millipore Sigma | 48-660MAG | |
| Mini LabRoller rotator | VWR | 10136-084 | |
| Phenylmethylsulfonyl fluoride | Sigma-Aldrich | P7626-1G | |
| Phosphate-buffered Saline (PBS) | VWR | 97064-158 | |
| Plate Sealer | VWR | 82050-992 | |
| Polypropylene microfuge tubes | VWR | 20901-547 | |
| Mini LabRoller | Millipore Sigma | Z674591 | |
| Reagent Reservoirs | VWR | 89094-668 | |
| R Programming Language | |||
| RStudio | www.rstudio.com | ||
| Sonicator | |||
| Titer plate shaker | VWR | 12620-926 | |
| Tween20 | Sigma-Aldrich | P9416-50ML | |
| 1 m acrylic guide tube | McMaster-Carr | 49035K85 | |
| 4 photon counting avalanche photodiode | Perkin-Elmer | SPCM-AQ4C-IO | |
| 400 um multimode source fiber | Thorlabs Inc. | FT-400-EMT | |
| 54 g bolt | Ace Hardware | 0.95 cm basic body diameter, 2 cm head diameter, 10.2 cm length | |
| 780 nm single mode detector fiber | Thorlabs Inc. | 780HP | |
| 852 nm long-coherence length laser | TOPTICA Photonics | iBeam smart |
Odniesienia
- Langlois, J. A., Rutland-Brown, W., Wald, M. M. The epidemiology and impact of traumatic brain injury: a brief overview. Journal of Head Trauma Rehabilitation. 21 (5), 375-378 (2006).
- Iraji, A., et al. Resting State Functional Connectivity in Mild Traumatic Brain Injury at the Acute Stage: Independent Component and Seed-Based Analyses. Journal of Neurotrauma. 32 (14), 1031-1045 (2015).
- Guskiewicz, K. M., et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. Journal of the American Medical Association. 290 (19), 2549-2555 (2003).
- Longhi, L., et al. Temporal window of vulnerability to repetitive experimental concussive brain injury. Neurosurgery. 56 (2), 364-374 (2005).
- Committee on Sports-Related Concussions in Youth, Board on Children, Youth, and Families, Institute of Medicine, National Research Council. . Sports-Related Concussions in Youth: Improving the Science, Changing the Culture. , (2014).
- Barkhoudarian, G., Hovda, D. A., Giza, C. C. The Molecular Pathophysiology of Concussive Brain Injury - an Update. Physical Medicine and Rehabilitation Clinics of North America. 27 (2), 373-393 (2016).
- McCrory, P., et al. Consensus statement on concussion in sport--the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Clinical Journal of Sport Medicine. 23 (2), 89-117 (2012).
- Belanger, H. G., Vanderploeg, R. D., Curtiss, G., Warden, D. L. Recent neuroimaging techniques in mild traumatic brain injury. Journal of Neuropsychiatry and Clinical Neurosciences. 19 (1), 5-20 (2007).
- Sours, C., Zhuo, J., Roys, S., Shanmuganathan, K., Gullapalli, R. P. Disruptions in Resting State Functional Connectivity and Cerebral Blood Flow in Mild Traumatic Brain Injury Patients. PLoS ONE. 10 (8), 0134019 (2015).
- Buckley, E. M., et al. Decreased Microvascular Cerebral Blood Flow Assessed by Diffuse Correlation Spectroscopy after Repetitive Concussions in Mice. Journal of Cerebral Blood Flow & Metabolism. 35 (12), 1995-2000 (2015).
- Sankar, S. B., et al. Low cerebral blood flow is a non-invasive biomarker of neuroinflammation after repetitive mild traumatic brain injury. Neurobiology of Disease. 124, 544-554 (2019).
- Vagnozzi, R., et al. Temporal window of metabolic brain vulnerability to concussions: mitochondrial-related impairment--part I. Neurosurgery. 61, 379-388 (2007).
- Longhi, L., et al. Temporal window of vulnerability to repetitive experimental concussive brain injury. Neurosurgery. 56, 364-374 (2005).
- Fujita, M., Wei, E. P., Povlishock, J. T. Intensity- and interval-specific repetitive traumatic brain injury can evoke both axonal and microvascular damage. Journal of Neurotrauma. 29, 2172-2180 (2012).
- Angoa-Perez, M., et al. Animal models of sports-related head injury: bridging the gap between preclinical research and clinical reality. Journal of Neurochemistry. 129, 916-931 (2014).
- Prins, M. L., Hales, A., Reger, M., Giza, C. C., Hovda, D. A. Repeat traumatic brain injury in the juvenile rat is associated with increased axonal injury and cognitive impairments. Developmental Neuroscience. 32, 510-518 (2010).
- Viano, D. C., Hamberger, A., Bolouri, H., Saljo, A. Concussion in professional football: animal model of brain injury--part 15. Neurosurgery. 64, 1162-1173 (2009).
- Kane, M. J., et al. A mouse model of human repetitive mild traumatic brain injury. Journal of Neuroscience Methods. 203, 41-49 (2012).
- Meehan, W. P., Zhang, J., Mannix, R., Whalen, M. J. Increasing Recovery Time Between Injuries Improves Cognitive Outcome After Repetitive Mild Concussive Brain Injuries in Mice. Neurosurgery. 71 (4), 885-892 (2012).
- Durduran, T., Yodh, A. G. Diffuse correlation spectroscopy for non-invasive, micro-vascular cerebral blood flow measurement. NeuroImage. 85, 51-63 (2014).
- Sathialingam, E., et al. Small separation diffuse correlation spectroscopy for measurement of cerebral blood flow in rodents. Biomedical Optics Express. 9 (11), 5719 (2018).
- Lee, S. Y., et al. Noninvasive optical assessment of resting-state cerebral blood flow in children with sickle cell disease. Neurophotonics. 6 (03), 1 (2019).
- Wang, H., Liu, Q., Tu, Y. Interpretation of partial least-squares regression models with VARIMAX rotation. Partial Least Squares. 48 (1), 207-219 (2005).
- Eriksson, L., Byrne, T., Johansson, E., Trygg, J., Vikström, C. Multi- and Megavariate Data Analysis Basic Principles and Applications. Umetrics Academy. , (2013).
- Conzen, P. F., et al. Systemic and regional hemodynamics of isoflurane and sevoflurane in rats. Anesthesia and Analgesia. 74 (1), 79-88 (1992).
- Durduran, T., Choe, R., Baker, W. B., Yodh, A. G. Diffuse optics for tissue monitoring and tomography. Reports on Progress in Physics. 73 (7), 076701 (2010).
- Lee, S. Y., et al. Small separation frequency-domain near-infrared spectroscopy for the recovery of tissue optical properties at millimeter depths. Biomedical Optics Express. 10 (10), 5362-5377 (2019).
- . plsRglm: Partial Least Squares Regression for Generalized Linear Models Available from: https://CRAN.R-project.org/package=pplsRglm (2019)
- White, B. R., Bauer, A. Q., Snyder, A. Z., Schlaggar, B. L., Lee, J. M., Culver, J. P. Imaging of functional connectivity in the mouse brain. PLoS One. 6, 16322 (2011).
- Buckley, E. M., Parthasarathy, A. B., Grant, P. E., Yodh, A. G., Franceschini, M. A. Diffuse correlation spectroscopy for measurement of cerebral blood flow: future prospects. Neurophotonics. 1 (1), 011009 (2014).
- Rowan, O., et al. Cerebrovascular reactivity measured in awake mice using diffuse correlation spectroscopy. Neurophotonics. 8 (1), (2021).
- Tate, J., Ward, G. Interferences in immunoassay. The Clinical Biochemist. Reviews. 25 (2), 105-120 (2004).
- Staples, E., Ingram, R. J. M., Atherton, J. C., Robinson, K. Optimising the quantification of cytokines present at low concentrations in small human mucosal tissue samples using Luminex assays. Journal of Immunological Methods. 394 (1-2), 1-9 (2013).
- Gierut, J. J., et al. Network-level effects of kinase inhibitors modulate TNF-α-induced apoptosis in the intestinal epithelium. Science Signaling. 8 (407), 129 (2015).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone