Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Electric and Magnetic Field Devices for Stimulation of Biological Tissues
W tym Artykule
Podsumowanie
This protocol describes the step-by-step process to build both electrical and magnetic stimulators used to stimulate biological tissues. The protocol includes a guideline to simulate computationally electric and magnetic fields and manufacture of stimulator devices.
Streszczenie
Electric fields (EFs) and magnetic fields (MFs) have been widely used by tissue engineering to improve cell dynamics such as proliferation, migration, differentiation, morphology, and molecular synthesis. However, variables such stimuli strength and stimulation times need to be considered when stimulating either cells, tissues or scaffolds. Given that EFs and MFs vary according to cellular response, it remains unclear how to build devices that generate adequate biophysical stimuli to stimulate biological samples. In fact, there is a lack of evidence regarding the calculation and distribution when biophysical stimuli are applied. This protocol is focused on the design and manufacture of devices to generate EFs and MFs and implementation of a computational methodology to predict biophysical stimuli distribution inside and outside of biological samples. The EF device was composed of two parallel stainless-steel electrodes located at the top and bottom of biological cultures. Electrodes were connected to an oscillator to generate voltages (50, 100, 150 and 200 Vp-p) at 60 kHz. The MF device was composed of a coil, which was energized with a transformer to generate a current (1 A) and voltage (6 V) at 60 Hz. A polymethyl methacrylate support was built to locate the biological cultures in the middle of the coil. The computational simulation elucidated the homogeneous distribution of EFs and MFs inside and outside of biological tissues. This computational model is a promising tool that can modify parameters such as voltages, frequencies, tissue morphologies, well plate types, electrodes and coil size to estimate the EFs and MFs to achieve a cellular response.
Wprowadzenie
EFs and MFs have been shown to modify cell dynamics, stimulating proliferation and increasing synthesis of the main molecules associated with the extracellular matrix of tissues1. These biophysical stimuli can be applied in different ways by using specific settings and devices. Regarding the devices to generate EFs, direct coupling stimulators use electrodes that are in contact with biological samples in vitro or implanted directly into tissues of patients and animals in vivo2; however, there are still limitations and deficiencies that include insufficient biocompatibility by the electrodes in contact, changes in the pH and molecular oxygen levels1. On the contrary, indirect coupling devices generate EFs between two electrodes, which are placed in parallel to biological samples3, allowing a non-invasive alternative technique to stimulate biological samples and avoid direct contact between tissues and electrodes. This type of device can be extrapolated to future clinical applications to perform procedures with minimal invasion to the patient. In relation to devices that generate MFs, inductive coupling stimulators create a time-varying electric current, which flows through a coil that is located around cell cultures4,5. Finally, there are combined devices, which use EFs and static MFs to generate transient electromagnetic fields1. Given that there are different configurations to stimulate biological samples, it is necessary to consider variables such as tension and frequency when biophysical stimuli are applied. Voltage is an important variable, since it influences the behavior of biological tissues; for instance, it has been shown that cell migration, orientation and gene expression depend on the amplitude of applied voltage3,6,7,8,9,10. Frequency plays an important role in biophysical stimulation, as it has been evidenced that these occur naturally in vivo. It has been demonstrated that high and low frequencies have beneficial effects on cells; especially, in cell membrane voltage-gated calcium channels or endoplasmic reticulum, which trigger different signaling-pathways at intracellular level1,7,11.
According to the abovementioned, a device for generating EFs consists of a voltage generator connected to two parallel capacitors12. This device was implemented by Armstrong et al. to stimulate both the proliferative rate and the molecular synthesis of chondrocytes13. An adaptation of this device was performed by Brighton et al. who modified cell culture well-plates by drilling their top and bottom lids. Holes were filled by cover slides, where the bottom glasses were used to culture biological tissues. Electrodes were placed on each cover slide to generate EFs14. This device was used to electrically stimulate chondrocytes, osteoblasts and cartilage explants, showing an increase in cell proliferation14,15,16 and molecular synthesis3,17. The device designed by Hartig et al. consisted of a wave generator and a voltage amplifier, which were connected to parallel capacitors. Electrodes were made of high-quality stainless-steel located in an insulating case. The device was used to stimulate osteoblasts, showing a significant increase in proliferation and protein secretion18. The device used by Kim et al. consisted of a biphasic current stimulator chip, which was built using a manufacturing process of complementary semiconductors of high-voltage metal oxide. A culture well-plate was designed to culture cells over a conductive surface with electrical stimulation. Electrodes were coated in gold over silicon plates19. This device was used to stimulate osteoblasts, showing an increase in the proliferation and the synthesis of the vascular endothelial growth factor19, and stimulating the production of alkaline phosphatase activity, calcium deposition and bone morphogenic proteins20. Similarly, this device was used to stimulate the proliferative rate and expression of vascular endothelial growth factor of human bone marrow mesenchymal stem cells21. The device designed by Nakasuji et al. was composed of a voltage generator connected to platinum plates. Electrodes were built to measure the electric potential at 24 different points. This device was used to stimulate chondrocytes, showing that EFs did not alter cell morphology and increased proliferation and molecular synthesis22. The device used by Au et al. consisted of a glass chamber equipped with two carbon rods connected to a cardiac stimulator with platinum wires. This stimulator was used to stimulate cardiomyocytes and fibroblasts, improving cell elongation and fibroblast alignment23.
Different MF devices have been manufactured based on Helmholtz coils to stimulate several types of biological samples. For instance, Helmholtz coils have been used to stimulate proliferation and molecular synthesis of chondrocytes24,25, enhance proteoglycan synthesis of articular cartilage explants26, improve gene upregulation related to bone formation of osteoblast-like cells27, and increase proliferation and molecular expression of endothelial cells28. Helmholtz coils generate MFs throughout two coils located one in front the other. The coils must be placed with a distance equal to the radius of the coils to ensure a homogeneous MF. The disadvantage of using Helmholtz coils lies in the coil dimensions, because they need to be big enough to generate the required MF intensity. Additionally, the distance between coils must be adequate to ensure a homogeneous distribution of MFs around biological tissues. To avoid issues caused by Helmholtz coils, different studies have been focused on solenoid coils manufacturing. Solenoid coils are based on a tube, which is wound with copper wire to generate MFs. Copper wire inputs can be connected directly to the outlet or a power supply to energize the coil and create MFs in the center of the solenoid. The more turns the coil has, the greater the MF generated. The MF magnitude also depends on the voltage and current applied to energize the coil29. Solenoid coils have been used to stimulate magnetically different kind of cells such as HeLa, HEK293 and MCF730 or mesenchymal stem cells31.
Devices used by different authors have not considered either the adequate size of electrodes or correct length of the coil to homogeneously distribute both EFs and MFs. Furthermore, devices generate fixed voltages and frequencies, limiting their use to stimulate specific biological tissues. For this reason, in this protocol a computational simulation guideline is performed to simulate both capacitive systems and coils to ensure homogeneous distribution of EFs and MFs over biological samples, avoiding the edge effect. Additionally, it is shown that the design of electronic circuits generate voltages and frequency between the electrodes and the coil, creating EFs and MFs that will overcome limitations caused by impedance of cell culture well-plates and air. These modifications will allow the creation of non-invasive and adaptive bioreactors to stimulate any biological tissue.
Protokół
1. Simulation of EFs and MFs
NOTE: Simulation of EFs and MFs was performed in COMSOL Multiphysics.
- Select an axisymmetric 2D configuration to represent both domains electric and magnetic.
- In the physic configuration, select either the Electric Current interface to compute EFs in parallel electrodes or the Magnetic Field interface to compute MFs around coils.
- In the study configuration, select Frequency Domain to compute the response of a linear or linearized model subjected to harmonic excitation for one or several frequencies.
- Once inside the interface to start building the model, follow the next steps according to the model of the interest.
- Building a model for EFs
- Create geometries. In the Model Builder, select Geometry. Then, locate the Units section and choose mm. On the Geometry Toolbar, select Rectangle and type the dimensions of each component in the Size and Shape box of the Rectangle Window settings . The geometry is composed by air, two parallel electrodes, a culture well-plate, culture media and a biological sample, which in this case is represented by a scaffold of hyaluronic acid - gelatin hydrogel (see dimensions of each element in Table 1). Once all geometries are built, click Build All Objects.
- Create selections. On the Definitions Toolbar, click Explicit to create a selection for the metal domain. Select the geometries that represent the electrodes. After, right-click on Explicit 1 to rename it. Type Metal in the new label text field.
- On the other hand, on the Definitions Toolbar, click Complement. Locate the Input Entities section in the Complement Settings window. Then, under Selections to invert, click Add and select Metal in the Selections to invert list from the Add dialog box. Thereafter, right-click in Complement 1 to rename it. Type Model domain in the new label text field.
- Create boundaries. Click Explicit on the Definitions Toolbar. After, locate the Input Entities section in the Settings window for Explicit and from the Geometric entity level list, choose Boundary. Here, select all boundaries for the bottom electrode. Right-click Explicit 2 to rename it. Type Ground boundaries in the new label text field. Repeat these steps but selecting all boundaries for the upper electrode. Thereafter, right-click Explicit 3 to rename it. Type Terminal boundaries in the new label text field.
- Add electric currents. In the Model Builder window, under Component 1 click Electric Currents (ec). Then, locate the Domain Selection section in the Electric Currents Settings window. From the Selection list, choose Model domain. On the Physics Toolbar, click Boundaries and choose Ground. After, locate the Boundary Selection section in the Ground Settings window and choose Ground boundaries from the Selection list.
- Thereafter, click Boundaries and choose Terminal on the Physics Toolbar. Finally, locate the Boundary Selection section in the Terminal Settings window and choose Terminal boundaries from the Selection list; here, locate the Terminal section and choose Voltage from the Terminal list and type 100 V.
- Add materials. Click Add Material on the Home Toolbar to open the Add Material window. Search air and stainless-steel and add them to the Model Builder window. Then, click Blank Material on the Home Toolbar and add three new blank materials for culture media, scaffold (hydrogel) and polystyrene (culture well-plate).
- Select a blank material to assign the dielectric properties. Locate the Material Properties list in the Material settings window and select relative permittivity and electric conductivity from the Basic Properties option list. The dielectric properties for culture media, hydrogel and culture well-plate are in Table 2. Repeat this procedure for all blank materials.
- Assign each material to the geometries previously built. Select the air material form the Model Builder window; then, select the domains that correspond to air from the Graphic window. Repeat this step for all materials created. Make sure that each domain corresponds to the correct material. To make sure that all materials are correctly assigned, click on each material from the Model Builder window and observe whether the domains are highlighted in blue within the Graphic window.
- Build mesh. Right-click Mesh 1 in the Model Builder window and select Free Triangular. Repeat this step by selecting Size. In the Mesh Setting window select Mesh Controlled by the User from the Sequence Type list. Then, expand the Mesh options in the Model Builder window and click Size.
- Locate Element Size Parameters in the Size Setting window and type 1 mm for maximum element size, 0.002 mm for minimum element size, 1.1 for maximum item growth rate, 0.2 for curvature factor and 1 for resolution of narrow regions. Then, expand the Mesh options in the Model Builder window and click Free Triangular 1. Here, select all domains to be meshed. Finally, click Build All in the Mesh Setting window.
- Create study. Click Study 1 in the Model Builder window. Then, locate the Study Settings section in the Study Settings window and clear the Generate default plots check box. Expand the Study 1 node in the Model Builder window and click Step 1: Frequency Domain. Finally, locate the Study Settings section in the Frequency Domain Settings window and type 60 kHz in the Frequencies text field.
- Calculate study. Click Show Default Solver on the Study toolbar. Then, expand the Study 1 Solver Configurations node in the Model Builder window. Expand the Solution 1 (sol1) node in the Model Builder window; thereafter, click Stationary Solver 1 in the Stationary Solver Settings window and locate the General section and type 1e-6 in the Relative Tolerance text field. Finally, click Compute on the Study Toolbar.
- Plot results. Select Results section on the Home toolbar and add 2D Plot Group. Then, right-click 2D Plot Group 1 in the Model Builder window and choose Surface. Then, locate the Data section in the Surface Settings window and select Precursor. After, locate the Expression section in the Surface Settings window; here, click in the plus (+) symbol to open a new window and locate the follow route from the selection list (Model - Component 1 - Electric Currents - Electric). Here, select ec.normE - EF Norm. Finally, click on Graphic in the Surface Settings window to plot the results.
- Building a model for MFs
- Create geometries. In the Model Builder, select Geometry; then, locate the Units section and choose mm. On the Geometry Toolbar select Rectangle and type the dimensions of each component in the Size and Shape box of the Rectangle Window Settings . The geometry is composed by air and cooper (see dimensions of each element in Table 1). Once all geometries are built, click Build All Objects.
- Add materials. Click Add Material on the Home Toolbar to open the Add Material window. Search air and copper and add them to the Model Builder window. The dielectric properties for copper are in Table 2.
- Create boundaries. Click Magnetics Field on the Model Builder window. Here, locate Equation list on the Magnetic Fields Settings window and choose Frequency Domain equation from the Equation Form list. In Frequency list choose From solver. After, locate Ampere's Law on the Magnetic Field list in the Model Builder window. In the type 293.15[K] in Temperature, 1[atm] in Absolute Pressure from the Inputs Model list. Then, choose Solid from the Material type list in the Ampere's Law Settings window. Make sure that Electric conductivity, Relative permittivity and Relative Permeability correspond to the From material in the list.
- Locate Axial Symmetry on the Magnetic Field list in the Model Builder window. Make sure that the axial symmetry line is highlighted in both Boundary Selection list and Graphic window. Then, locate Magnetic Isolation on the Magnetic Field list in the Model Builder window. Make sure that boundaries from the geometry are highlighted in both Boundary Selection list and Graphic window.
- Locate Initial Values on the Magnetic Field list in the Model Builder window. Select geometries previously built and include them in the Domain Selection from the Initial Values Settings window.
- Introduce coil features. Locate Multiple Coil on the Magnetic Field list in the Model Builder window. Here, selects the geometry that represents the coil and include them in the Domain Selection from the Multiple Coil Settings window.
- Locate the Multiple Coil list on the Multiple Coil Setting window; here, locate Coil excitation list and select Current; thereafter, type 1[A] in the Coil current list, 450 in the Number of turns and 6e7[S/m] in the Coil conductivity.
- Locate the Coil wire cross-sectional area and choose North American cable diameter (Brown & Sharpe) from the list and type 18 in the AWG option. Make sure that Relative permittivity and Relative Permeability correspond to From material in the list.
- Build mesh. In the Mesh Setting window select Mesh Controlled by the physics from the Sequence Type list. After, locate Element Size Parameters in the Mesh Setting window and select Extremely fine. Finally, select all domains to be meshed and click Build All in the Mesh Setting window.
- Create study. Click Study 1 in the Model Builder window. Then, locate the Study Settings section in the Study Settings window and clear the Generate default plots check box. Expand the Study 1 node in the Model Builder window and click Step 2: Frequency Domain. Finally, locate the Study Settings section in the Frequency Domain Settings window and type 60 Hz in the Frequencies text field.
- Calculate study. Click Show Default Solver on the Study toolbar. Then, expand the Study 1 Solver Configurations node in the Model Builder window. Expand the Solution 1 (sol1) node in the Model Builder window; thereafter, click Stationary Solver 1 in the Stationary Solver Settings window and locate the General section and type 1e-6 in the Relative tolerance text field. Finally, click Compute on the Study Toolbar.
- Plot results. Select Results section on the Home toolbar and add 2D Plot Group. Then, right-click 2D Plot Group 1 in the Model Builder window and choose Surface. Then, locate the Data section in the Surface Settings window and select Precursor.
- Locate the Expression section in the Surface Settings window. Here, click in the plus (+) symbol to open a new window and locate the follow route from the selection list (Model - Component 1 - Magnetic Field - Magnetic). Here, select mf.normB - Magnetic flux density Norm. Finally, click on Graphic in the Surface Settings window to plot the results.
- Building a model for EFs
2. Design and manufacturing of the electrical and magnetic stimulation devices
- The electrical stimulator device
NOTE: It is composed by a circuit based on the Wien Bridge Oscillator and two parallel stainless-steel electrodes. The circuit is a RC oscillator of phase shift, which uses a positive and negative feedback. The Wien Bridge Oscillator is composed by a lead-lag network, which divides the input voltage by the combination of two arms of the bridge: a resistor R5 with a capacitor C2 in series, and a resistor R6 with a capacitor C3 in parallel (Figure 1A). These components modulate the frequency of the oscillator. To build the electrical stimulator device follow the next steps:- Calculate the frequency using the resonant frequency equation (1).
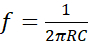
Where R = R5 = R6 are resistors and C = C2 = C3 are capacitors. Both R and C are placed in the two arms of the bridge (Figure 1A). Use R5 = R6 = 2.6 kΩ and C2 = C3 = 1 nF to obtain a frequency of 60 kHz. Resistors and capacitors may be calculated if a different frequency is required. - Design the circuit in such a way that voltage gain of the amplifier automatically compensates the amplitude changes of the output signal. In Figure 1A it is possible to observe the scheme of the circuit, while in Table of Materials section are listed the electronic components to build the circuit.
- Calculate the combination of resistors to generate the four output voltages. As shown in Figure 1A, use a combination of resistors R11, R12, R13 and R14 (equivalent resistance of 154 Ω) to generate a voltage of 50 Vp-p; resistors R17, R18 and R19 in series (equivalent resistance of 47,3 Ω) to obtain a voltage of 100 Vp-p; resistors R9 and R10 in series (equivalent resistance of 25,3 Ω) to generate a voltage of 150 Vp-p; and a combination of resistors R15 and R16 (equivalent resistance of 16,8 Ω) to obtain a voltage of 200 Vp-p.
- Use a transistor (TIP 31C) and a ferrite core transformer to implement a signal amplification stage. A toroidal ferrite core was used to wind an AWG 24 copper wire, completing a relation 1:200. Use two capacitors (C4 and C5) of 100 nF in parallel before the transformer to rectify the signal (Figure 1A).
- Prepare the PCB using a third-party PCB manufacturing service. The schematic diagram of the circuit is provided in Figure 1. Place all components on the PCB with antistatic tweezers. Use tin solder and soldering iron to solder all components.
- Manufacture a plastic case with input connectors to protect the circuit. Implement three input connectors to energize the circuit (12 V, -12 V and ground). Use two input connectors to connect the electrodes. Include three switches to change the resistors combination to obtain the four output voltages. Assemble the electronic circuit into the plastic case (Figure 1B).
- Manufacture two parallel stainless-steel electrodes (200 x 400 x 2 mm) and solder input connectors to each edge. The electrodes are located over Teflon or acrylic supports to eliminate any contact with the metal surface of the incubator (Figure 1C).
- Use an autoclave at 394.15 K (121 °C) for 30 minutes to sterilize the electrodes and use ultraviolet over night to sterilize the wires that are in contact with the incubator.
- Test the electrical stimulation device. Adjust the power supply in series to generate an output voltage of +12 V and -12 V between the ground and positive and negative terminals. Verify the output voltage of the power supply with a multimeter. Connect each output of the power supply in the correct input of the electrical stimulator (+12 V, -12 V and ground). Connect each electrode in the correct input connector of the electrical stimulator. The polarity is not important, as we are working on AC current. Place a culture well-plate between of the electrodes and verify the output signal with an oscilloscope. Adjust the switches of the electrical stimulator to generate the four output voltages (50, 100, 150 and 200 Vp-p).
- Safety recommendations. To avoid any issue when transferring or removing the electrodes from the incubator make sure that cables are not tangled. Disconnect cables from the oscillator before removing the electrodes from the incubator. Never place the electrodes without the acrylic or Teflon supports.
- Calculate the frequency using the resonant frequency equation (1).
- The magnetic stimulator device
- Estimate the number of turns to guarantee a homogeneous MF inside the coil using the equation (2), that describes the MF inside a solenoid coil.
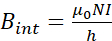
where μ0 is the magnetic permeability of the vacuum (4π×10-7), N is the number of turns of the copper wire, I is the current, and h, which should be greater than its diameter, is the length of the solenoid coil. - Determine the number of turns by choosing a length (h) of 250 mm, current of 1 A and a Bint = 2mT.
- Manufacture the coil. Build a polyvinyl chloride (PVC) tube with a length of 250 mm and a diameter of 84 mm to wind an AWG 18 copper wire completing 450 turns (Figure 2A). Dimensions were chosen based on the available space inside the incubator.
- Manufacture a cell culture well-plate support. Build a polymethyl methacrylate (PMMA) support to ensure that well-plates of 35 mm were always located in the middle of the coil where MFs are homogeneous (Figure 2A).
- Manufacture a transformer to increase the current of the circuit. Build a transformer with an output of 1 A - 6 V AC to reach a maximum MF of 2 mT. The input voltage of the transformer was 110 V AC at 60 Hz. These parameters correspond to the output voltage and frequency of a South America outlet.
- Connect the circuit. The transformer is connected directly to the outlet. Use a variable resistor (rheostat) to vary the current and generate MFs from 1 to 2 mT. Connect a fuse to protect the circuit (Figure 2B).
- Use ultraviolet over night to sterilize the wires that are in contact with the incubator. Wrap the coil with transparent stretch film and use ethanol to sterilize the coil.
- Test the MF device. Use a teslameter to measure the MF magnitude inside the coil. The teslameter probe was located in the center of the coil, allowing the measurement of MFs and currents simultaneously.
- Vary the MF magnitude. Use a rheostat to modify the resistance of the circuit (Figure 2B). A resistance value of 0.7 Ω was used to generate MFs of 1 mT.
- Safety recommendations. To avoid any issue when transferring or removing the solenoid from the incubator make sure that cables are not tangled. Disconnect cables from the transformer before removing the solenoid from the incubator. Never place the solenoid without the PMMA support. Firmly grasp both PMMA support from the base and the solenoid when transferring or removing from the incubator.
- Estimate the number of turns to guarantee a homogeneous MF inside the coil using the equation (2), that describes the MF inside a solenoid coil.
Wyniki
Computational simulation
Distributions of EFs and MFs are shown in Figure 3. On the one hand, it was possible to observe the homogeneous distribution of EFs in the capacitive system (Figure 3A). The EF was plotted to observe in detail the magnitude of the field inside the biological sample (Figure 3B). This simulation was useful to parametrize the size of the electrodes and manufacture them to avoid the edge e...
Dyskusje
Treatments used to heal different pathologies that affect human tissues are pharmacological therapies32 or surgical interventions33, which seek to relieve pain locally or replace affected tissues with explants or transplants. Recently, autologous cell therapy has been proposed as an alternative therapy to treat injured tissues, where cells are isolated from patient and expanded, through in vitro techniques, to be implanted at the site of the injury34...
Ujawnienia
The authors declare that they have no conflict of interest.
Podziękowania
The authors thank the financial support provided by "Fondo Nacional de Financiamiento para la Ciencia, la Tecnología, y la Innovación -Fondo Francisco José de Caldas- Minciencias" and Universidad Nacional de Colombia through the grant No. 80740-290-2020 and the support received by Valteam Tech - Research and Innovation for providing the equipment and technical support in the edition of the video.
Materiały
| Name | Company | Catalog Number | Comments |
| Electrical stimulator | |||
| Operational amplifier | Motorola | LF-353N | ---- Quantity: 1 |
| Resistors | ---- | ---- | 22 kΩ Quantity: 1 |
| Resistors | ---- | ---- | 10 kΩ Quantity: 3 |
| Resistors | ---- | ---- | 2.6 kΩ Quantity: 2 |
| Resistors | ---- | ---- | 2.2 kΩ Quantity: 1 |
| Resistors | ---- | ---- | 1 kΩ Quantity: 1 |
| Resistors | ---- | ---- | 220 Ω Quantity: 2 |
| Resistors | ---- | ---- | 22 Ω Quantity: 5 |
| Resistors | ---- | ---- | 10 Ω Quantity: 1 |
| Resistors | ---- | ---- | 6.8 Ω Quantity: 1 |
| Resistors | ---- | ---- | 3.3 Ω Quantity: 2 |
| Polyester capacitors | ---- | ---- | 1 nF Quantity: 2 |
| Polyester capacitors | ---- | ---- | 100 nF Quantity: 1 |
| VHF Band Amplifier Transistor JFET | Toshiba | 2SK161 | ---- Quantity: 1 |
| Power transistor BJT NPN | Mospec | TIP 31C | ---- Quantity: 1 |
| Zener diode | Microsemi | 1N4148 | ---- Quantity: 1 |
| Switch | Toogle Switch | SPDT - T13 | ---- Quantity: 3 |
| Toroidal ferrite core | Caracol | ---- | T*22*14*8 Quantity: 1 |
| Cooper wire | Greenshine | ---- | AWG – 24 Quantity: 1 |
| Relimate header with female housing | ADAFRUIT | ---- | 8 pin connectors Quantity: 1 |
| Relimate header with female housing | ADAFRUIT | ---- | 2 pin connectors Quantity: 1 |
| Female plug terminal connector | JIALUN | ---- | 4mm Lantern Plugs (Plug + Socket) 15 A Quantity: 1 |
| Aluminum Heat Sink | AWIND | ---- | For TIP 31C transistor Quantity: 1 |
| Led | CHANZON | ---- | 5 mm red Quantity: 1 |
| Integrated circuit socket connector | Te Electronics Co., Ltd. | ---- | Double row 8-pin DIP Quantity: 1 |
| 3 pin connectors set | STAR | ---- | JST PH 2.0 Quantity: 3 |
| 2 pin screw connectors | STAR | ---- | For PCB Quantity: 1 |
| 3 pin screw connectors | STAR | ---- | For PCB Quantity: 1 |
| Banana connector test lead | JIALUN | ---- | P1041 - 4 mm - 15 A Quantity: 7 |
| Bullet connectors to banana plug charge lead | JIALUN | ---- | 4 mm male-male/female-female adapters - 15 A Quantity: 1 |
| Case | ---- | ---- | ABS Quantity: 1 |
| Electrodes | ---- | ---- | Stainless – steel Quantity: 2 |
| Electrode support | ---- | ---- | Teflon Quantity: 2 |
| Printed circuit board | Quantity: 1 | ||
| Magnetic stimulator | |||
| Cooper wire | Greenshine | ---- | AWG – 18 Quantity: 1 |
| AC power plugs | ---- | ---- | 120 V AC – 60 Hz Quantity: 1 |
| Banana female connector test lead | JIALUN | ---- | 1Set Dual Injection - 4 mm – 15 A Quantity: 2 |
| Banana male connector test lead | JIALUN | ---- | 1Set Dual Injection - 4 mm 15 A Quantity: 1 |
| Cell culture well plate support | ---- | ---- | PMMA Quantity: 1 |
| Fuse | Bussmann | 2A | ---- Quantity: 1 |
| Transformer | ---- | ---- | 1A – 6 V AC Quantity: 1 |
| Tube | ---- | ---- | PVC Quantity: 1 |
| Variable rheostat | MCP | BXS150 | 10 Ω Quantity: 1 |
| General equipment | |||
| Digital dual source | PeakTech | DG 1022Z | 2 x 0 - 30 V / 0 - 5 A CC / 5 V / 3 A fijo Quantity: 1 |
| Digital Oscilloscope | Rigol | DS1104Z Plus | 100 MHz, bandwidth, 4 channels Quantity: 1 |
| Digital multimeter | Fluke | F179 | Voltage CC – CA (1000 V). Current CC – CA 10 A. Frequency 100 kHz Quantity: 1 |
Odniesienia
- Balint, R., Cassidy, N. J., Cartmell, S. H. Electrical Stimulation: A Novel Tool for Tissue Engineering. Tissue Engineering Part B: Reviews. 19 (1), 48-57 (2013).
- Ercan, B., Webster, T. J. The effect of biphasic electrical stimulation on osteoblast function at anodized nanotubular titanium surfaces. Biomaterials. 31 (13), 3684-3693 (2010).
- Brighton, C., Wang, W., Clark, C. The effect of electrical fields on gene and protein expression in human osteoarthritic cartilage explants. The Journal of Bone and Joint Surgery-American. 90 (4), 833-848 (2008).
- Baerov, R. M., Morega, A. M., Morega, M. Analysis of magnetotherapy effects for post-traumatic recovery of limb fractures. Revue Roumaine des Sciences Techniques- Série électrotechnique et énergétique. 65 (1-2), 145-150 (2020).
- Escobar, J. F., et al. In Vitro Evaluation of the Effect of Stimulation with Magnetic Fields on Chondrocytes. Bioelectromagnetics. 41 (1), 41-51 (2019).
- Brighton, C., Wang, W., Clark, C. Up-regulation of matrix in bovine articular cartilage explants by electric fields. Biochemical and Biophysical Research Communications. 342 (2), 556-561 (2006).
- Xu, J., Wang, W., Clark, C., Brighton, C. Signal transduction in electrically stimulated articular chondrocytes involves translocation of extracellular calcium through voltage-gated channels. Osteoarthritis and Cartilage. 17 (3), 397-405 (2009).
- Xia, Y., et al. Magnetic field and nano-scaffolds with stem cells to enhance bone regeneration. Biomaterials. 183, 151-170 (2018).
- Richter, A., Bartoš, M., Ferková, &. #. 3. 8. 1. ;. Physical Analysis of Pulse Low-Dynamic Magnetic Field Applied in Physiotherapy BT. World Congress on Medical Physics and Biomedical Engineering 2018. , 239-245 (2019).
- Miyakoshi, J. Effects of static magnetic fields at the cellular level. Progress in Biophysics and Molecular Biology. 87, 213-223 (2005).
- Zhang, K., Guo, J., Ge, Z., Zhang, J. Nanosecond Pulsed Electric Fields (nsPEFs) Regulate Phenotypes of Chondrocytes through Wnt/β-catenin Signaling Pathway. Scientific Reports. 4 (5836), 1-8 (2014).
- Brighton, C. T., Unger, A. S., Stambough, J. L. In vitro growth of bovine articular cartilage chondrocytes in various capacitively coupled electrical fields. Journal of Orthopaedic Research. 2 (1), 15-22 (1984).
- Armstrong, P. F., Brighton, C., Star, A. M. Capacitively coupled electrical stimulation of bovine growth plate chondrocytes grown in pellet form. Journal of Orthopaedic Research. 6 (2), 265-271 (1988).
- Brighton, C., Townsend, P. Increased cAMP production after short-term capacitively coupled stimulation in bovine growth plate chondrocytes. Journal of Orthopaedic Research. 6 (4), 552-558 (1988).
- Brighton, C. T., Jensen, L., Pollack, S. R., Tolin, B. S., Clark, C. Proliferative and synthetic response of bovine growth plate chondrocytes to various capacitively coupled electrical fields. Journal of Orthopaedic Research. 7 (5), 759-765 (1989).
- Brighton, C. T., Okereke, E., Pollack, S. R., Clark, C. In vitro bone-cell response to a capacitively coupled electrical field. The role of field strength, pulse pattern, and duty cycle. Clinical Orthopaedics and Related Research. 285, 255-262 (1992).
- Wang, W., Wang, Z., Zhang, G., Clark, C., Brighton, C. T. Up-regulation of chondrocyte matrix genes and products by electric fields. Clinical Orthopaedics and Related Research. 427, 163-173 (2004).
- Hartig, M., Joos, U., Wiesmann, H. P. Capacitively coupled electric fields accelerate proliferation of osteoblast-like primary cells and increase bone extracellular matrix formation in vitro. European Biophysics Journal. 29 (7), 499-506 (2000).
- Kim, I. S., et al. Biphasic electric current stimulates proliferation and induces VEGF production in osteoblasts. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1763 (9), 907-916 (2006).
- Kim, I., et al. Novel Effect of Biphasic Electric Current on In Vitro Osteogenesis and Cytokine Production in Human Mesenchymal Stromal Cells. Tissue Engineering Part A. 15, 2411-2422 (2009).
- Kim, I., et al. Novel action of biphasic electric current in vitro osteogenesis of human bone marrow mesenchymal stromal cells coupled with VEGF production. Bone. 43, 43-44 (2008).
- Nakasuji, S., Morita, Y., Tanaka, K., Tanaka, T., Nakamachi, E. Effect of pulse electric field stimulation on chondrocytes. Asian Pacific Conference for Materials and Mechanics. 1, 13-16 (2009).
- Au, H. T. H., Cheng, I., Chowdhury, M. F., Radisic, M. Interactive effects of surface topography and pulsatile electrical field stimulation on orientation and elongation of fibroblasts and cardiomyocytes. Biomaterials. 28 (29), 4277-4293 (2007).
- Vanessa, N., et al. In vitro exposure of human chondrocytes to pulsed electromagnetic fields. European Journal of Histochemistry. 51 (3), 203-211 (2007).
- Pezzetti, F., et al. Effects of pulsed electromagnetic fields on human chondrocytes: An in vitro study. Calcified Tissue International. 65 (5), 396-401 (1999).
- De Mattei, M., et al. Effects of electromagnetic fields on proteoglycan metabolism of bovine articular cartilage explants. Connective Tissue Research. 44 (3-4), 154-159 (2003).
- Sollazzo, V., Massari, L., Caruso, A., Mattei, M., Pezzetti, F. Effects of Low-Frequency Pulsed Electromagnetic Fields on Human Osteoblast-Like Cells In Wtro. Electromagnetobiology. 15, 75-83 (2009).
- Martino, C. F., Perea, H., Hopfner, U., Ferguson, V. L., Wintermantel, E. Effects of weak static magnetic fields on endothelial cells. Bioelectromagnetics. 31 (4), 296-301 (2010).
- Wada, K., et al. Design and implementation of multi-frequency magnetic field generator producing sinusoidal current waveform for biological researches. 2016 18th European Conference on Power Electronics and Applications (EPE'16 ECCE Europe). 2016, 1-8 (2016).
- Cho, H., Kim, S., Kim, K. K., Kim, K., Kim, K. Pulsed Electromagnetic Fields Stimulate Cellular Proliferation in Different Types of Cells. IEEE Transactions on Magnetics. 52 (7), 1-4 (2016).
- Yan, J., Dong, L., Zhang, B., Qi, N. Effects of extremely low-frequency magnetic field on growth and differentiation of human mesenchymal stem cells. Electromagnetic Biology and Medicine. 29 (4), 165-176 (2010).
- Enoch, S., Grey, J. E., Harding, K. G. ABC of wound healing. Non-surgical and drug treatments. BMJ. 332 (7546), 900-903 (2006).
- Bhosale, A. M., Richardson, J. B. Articular cartilage: Structure, injuries and review of management. British Medical Bulletin. 87 (1), 77-95 (2008).
- Al Hamed, R., Bazarbachi, A. H., Malard, F., Harousseau, J. -. L., Mohty, M. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer Journal. 9 (4), 44 (2019).
- Massari, L., et al. Biophysical stimulation of bone and cartilage: state of the art and future perspectives. International Orthopaedics. 43 (3), 539-551 (2019).
- Naskar, S., Kumaran, V., Basu, B. Reprogramming the Stem Cell Behavior by Shear Stress and Electric Field Stimulation: Lab-on-a-Chip Based Biomicrofluidics in Regenerative Medicine. Regenerative Engineering and Translational Medicine. 5 (2), 99-127 (2019).
- Hunckler, J., de Mel, A. A current affair: electrotherapy in wound healing. Journal of Multidisciplinary Healthcare. 10, 179-194 (2017).
- Henry, S. L., Concannon, M. J., Yee, G. J. The effect of magnetic fields on wound healing: experimental study and review of the literature. Eplasty. 8, 393-399 (2008).
- Hiemer, B., et al. Effect of electric stimulation on human chondrocytes and mesenchymal stem cells under normoxia and hypoxia. Molecular Medicine Reports. 18 (2), 2133-2141 (2018).
- Chao, P. H., et al. Chondrocyte translocation response to direct current electric fields. Journal of Biomechanical Engineering. 122 (3), 261-267 (2000).
- Zhao, M., Bai, H., Wang, E., Forrester, J., McCaig, C. Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. Journal of Cell Science. 117 (3), 397-405 (2004).
- Li, X., Kolega, J. Effects of direct current electric fields on cell migration and actin filament distribution in bovine vascular endothelial cells. Journal of Vascular Research. 39 (5), 391-404 (2002).
- Singh, B., Dixit, A. Multistage amplifier and tuned amplifier. Analog Electronics. , 87-131 (2007).
- Esfandiari, E., et al. The effect of high frequency electric field on enhancement of chondrogenesis in human adipose-derived stem cells. Iranian Journal Basic Medical Sciences. 4 (3), 571-576 (2014).
- Mardani, M., et al. Induction of chondrogenic differentiation of human adipose-derived stem cells by low frequency electric field. Advanced Biomedical Research. 5 (97), 1-7 (2016).
- Karaman, O., Gümüşay, M., Demirci, E. A., Kaya, A. Comparative assessment of pulsed electromagnetic fields (PEMF) and pulsed radio frequency energy (PRFE) on an in vitro wound healing model. International Journal of Applied Electromagnetics and Mechanics. 57, 427-437 (2018).
- Glinka, M., et al. Test chambers for cell culture in static magnetic field. Journal of Magnetism and Magnetic Materials. 331, 208-215 (2013).
- Vacek, T. P., et al. Electrical stimulation of cardiomyocytes activates mitochondrial matrix metalloproteinase causing electrical remodeling. Biochemical and Biophysical Research Communications. 404 (3), 762-766 (2011).
- Okutsu, S., et al. Electric Pulse Stimulation Induces NMDA Glutamate Receptor mRNA in NIH3T3 Mouse Fibroblasts. The Tohoku Journal of Experimental Medicine. 215 (2), 181-187 (2008).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone