Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Evaluation of the Efficacy of Organic Peroxyacids for Eradicating Dairy Biofilms Using an Approach Combining Static and Dynamic Methods
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
This protocol describes an approach combining static and dynamic methods to evaluate the efficacy of organic peroxyacids for eradicating biofilms in the dairy industry. This approach may also be used to test the effectiveness of new biological or chemical formulations for controlling biofilms.
Streszczenie
The presence of biofilms in the dairy industry is of major concern, as they may lead to the production of unsafe and altered dairy products due to their high resistance to most clean-in-place (CIP) procedures frequently used in processing plants. Therefore, it is imperative to develop new biofilm control strategies for the dairy industry. This protocol is aimed at evaluating the efficacy of organic peroxyacids (peracetic, perpropionic, and perlactic acids and a commercial peracetic acid-based disinfectant) for eradicating dairy biofilms using a combination of static and dynamic methods. All the disinfectants were tested on the strongest biofilm-producing bacteria in either a single or a mixed biofilm using the minimum biofilm eradication concentration (MBEC) assay, a static high-throughput screening method. A contact time of 5 min with the disinfectants at the recommended concentrations successfully eradicated both the single and mixed biofilms. Studies are currently ongoing to confirm these observations using the Center for Disease Control (CDC) biofilm reactor, a dynamic method to mimic in situ conditions. This type of bioreactor enables the use of a stainless-steel surface, which constitutes most industrial equipment and surfaces. The preliminary results from the reactor appear to confirm the efficacy of organic peroxyacids against biofilms. The combined approach described in this study may be used to develop and test new biological or chemical formulations for controlling biofilms and eradicating microorganisms.
Wprowadzenie
The dairy industry is a major industrial sector worldwide, including in Canada, where there are more than 10,500 dairy farms producing nearly 90 million hL of milk each year1. Despite the strict hygiene requirements imposed in the dairy industry, including in processing plants, milk constitutes a great culture medium for microorganisms, and thus, dairy products are likely to contain microorganisms, including spoilage or pathogenic microorganisms. These pathogens can cause various diseases; for example, Salmonella sp. and Listeria monocytogenes can cause gastroenteritis and meningitis, respectively2. Spoilage microorganisms can affect the quality and organoleptic properties of dairy products by producing gases, extracellular enzymes, or acids3. The appearance and color of the milk may also be altered, for example by Pseudomonas spp.4.
Some of these microorganisms can form biofilms on different surfaces, including stainless steel. Such biofilms enable the persistence and multiplication of microorganisms on the surface of the equipment and, thus, the contamination of the dairy products5. Biofilms are also problematic because of their ability to impede heat transfer and accelerate the corrosion of the equipment, leading to premature replacement of the equipment and, thus, to economic losses6.
Clean-in-place (CIP) procedures allow the food industry to control the growth of microorganisms. These procedures involve the sequential use of sodium hydroxide, nitric acid, and, sometimes, sanitizers containing hypochlorous acid and peracetic acid7,8. Although hypochlorous acid is highly effective against microorganisms, it also reacts with natural organic matter, causing the formation of toxic by-products9. Peracetic acid does not generate harmful by-products10; however, its effectiveness against biofilms in the food industry is highly variable10,11. Recently, other peroxyacids, including perpropionic and perlactic acids, have been studied for their antimicrobial activity, and they appear to be a good alternative for the control of microbial growth in biofilms12,13.
Therefore, this study aimed to evaluate the efficacy of organic peroxyacids (peracetic, perpropionic, and perlactic acids and a peracetic acid-based disinfectant) for eradicating dairy biofilms using an approach combining the minimum biofilm eradication concentration (MBEC) assay, a static high-throughput screening method, and the Center for Disease Control (CDC) biofilm reactor, a dynamic method that mimics in situ conditions. The MBEC assay is hereafter referred to as "biofilm microtiter plates" in the protocol. The protocol presented here and the representative results demonstrate the efficacy of organic peroxyacids and their potential application for controlling microbial biofilms in the dairy industry.
Protokół
The work contained in this article requires a biosafety level 2 laboratory and was previously approved (Project number 119689) by the Université Laval institutional biosafety committee.
NOTE: The flowchart in Figure 1 represents a summary of the methodology combining static and dynamic approaches that was used to evaluate the efficacy of organic peroxyacids for eradicating biofilms.
1. Preparation of materials
- Microbial isolates
- Turn on the biological safety cabinet (BSC) 15 min before use, and clean it with a 70% (v/v) alcohol solution.
- Once the BSC is sterile, place a vial of the microbial isolate to be tested (Pseudomonas azotoformans or Brevundimonas vesicularis in this study), an inoculating loop, a 15 mL tube filled with 10 mL of sterile tryptic soy broth (TSB), and a vortex mixer. Before placement in the BSC, disinfect all the materials with alcohol.
- Vortex the vial of microbial isolate to homogenize the culture.
- Aseptically transfer 20 µL of the microbial isolate into 10 mL of sterile TSB contained in a 15 mL tube and incubate it at 30 °C for 16-24 h with agitation at 160 rpm.
CAUTION: B. vesicularis must be used in a containment level 2 laboratory in accordance with the guidelines required for handling pathogenic organisms. The handler must be properly trained and must wear safety glasses, gloves, and a coat.
- Disinfectants
- To prepare the organic peroxyacid solution (60 mL), add 24 mL of hydrogen peroxide and 36 mL of acid (acetic, propionic, or lactic acid) into a 250 mL Erlenmeyer flask. Then, add a predefined volume of 10 M sulfuric acid (655 µL for peracetic acid, 635 µL for perpropionic acid, or 715 µL for perlactic acid). Gently shake the flask to mix, and put the flask into a 30 °C water bath set up inside the chemical hood. Incubate the flask for 2 days, with gentle mixing every morning.
NOTE: The commercial peracetic acid-based disinfectant (see Table of Materials) was directly provided by the manufacturer.
CAUTION: The disinfectants must be used under a chemical hood. Safety glasses and gloves must be worn for the duration of the experiment. For more information about the disinfectants, please refer to the respective Material Safety Data Sheet. - Perform titration of hydrogen peroxide as described below.
- Place an empty 300 mL beaker on an analytical balance (see Table of Materials) and tare the scale. Weigh approximately 0.23 g of disinfectant into the beaker and note the exact weight added. Add 100 g of cold 1 N sulfuric acid solution into the beaker, add a magnetic stir bar into the beaker, and place it on a stir plate.
- Let the solution stir until complete homogenization. Then, add three drops of a ferroin indicator solution (0.1 wt% in H2O) into the beaker, and titrate with 0.1 N cerium sulfate solution until the solution changes from a salmon-pink color to a light-blue color. Note the volume of cerium sulfate solution added.
- Calculate the percentage of hydrogen peroxide using the following formula:
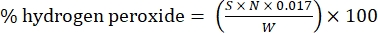 Eq. (1)
Eq. (1)
where S is the volume of cerium sulfate solution added, N is the normality of the cerium sulfate solution (0.1 N), W is the weight of the sample (~0.2300 g), and 0.017 = (1 mol H2O2/2 mol Ce) × (34.0147 g H2O2 /1 mol H2O)2 × (1 L/1,000 mL)
- Perform titration of the organic peroxyacids as described below.
- Add 20 mL of 7.5% (w/v) potassium iodide solution into a beaker. Titrate slowly with 0.1 N sodium thiosulfate solution until the blue color of the solution begins to turn a pale brown/orange.
- Add 2 mL of starch solution (1 wt% in H2O) to the beaker and titrate with 0.1 N sodium thiosulfate solution until the solution changes from black to orange. Note the volume of sodium thiosulfate solution used.
- Calculate the percentage of organic peroxyacid with the following formula:
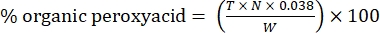 Eq. (2)
Eq. (2)
where T is the volume of sodium thiosulfate solution used, N is the normality of the sodium thiosulfate solution (0.1 N), W is the weight of the sample (~0.2300g), and 0.038 = (1 mol CH3COOOH/1 mol I2) × (1 mol I2/2 mol S2O3) × (76.06 g/1 mol CH3COOOH) × (1 L/1,000 mL)
NOTE: Repeat step 1.2.2 and step 1.2.3 with two more samples to perform each test in triplicate.
- To prepare the organic peroxyacid solution (60 mL), add 24 mL of hydrogen peroxide and 36 mL of acid (acetic, propionic, or lactic acid) into a 250 mL Erlenmeyer flask. Then, add a predefined volume of 10 M sulfuric acid (655 µL for peracetic acid, 635 µL for perpropionic acid, or 715 µL for perlactic acid). Gently shake the flask to mix, and put the flask into a 30 °C water bath set up inside the chemical hood. Incubate the flask for 2 days, with gentle mixing every morning.
2. Formation of single and mixed biofilms
- Biofilm microtiter plates
- Vortex the tube containing the bacterial culture (20 µL of the strain + 10 mL of TSB medium, prepared in step 1.1.4). Perform serial dilution and plating on tryptic soy agar (TSA) to determine the bacterial cell count (cfu) of the overnight culture. Then, aseptically transfer 100 µL of the culture into 10 mL of sterile TSB medium (for a final concentration of approximately 2 x 107 cfu/mL).
NOTE: For the assay with the bioreactor, a volume of 100 µL of the microbial isolate is transferred into 100 mL of sterile TSB. - Vortex the tube. For each bacterium, transfer the diluted bacterial culture to the biofilm microtiter plate (150 µL per well) in triplicate using a multichannel pipette. Load 150 µL of TSB medium into three new wells to serve as controls. Incubate the biofilm microtiter plate (see Table of Materials) at 30 °C for 24 h without agitation.
NOTE: For mixed biofilm assays, add 75 µL of each suspension for a total volume of 150 µL. The biofilm microtiter plate contains pegs in its lids, on which the biofilms form.
- Vortex the tube containing the bacterial culture (20 µL of the strain + 10 mL of TSB medium, prepared in step 1.1.4). Perform serial dilution and plating on tryptic soy agar (TSA) to determine the bacterial cell count (cfu) of the overnight culture. Then, aseptically transfer 100 µL of the culture into 10 mL of sterile TSB medium (for a final concentration of approximately 2 x 107 cfu/mL).
- Bioreactor
- Clean and air-dry the parts of the bioreactor (see Table of Materials) following the manufacturer's instructions, and proceed to prepare the reactor as described below.
- First, place the flat blade inside the 1 L glass beaker (bioreactor), which is attached to its holder by a magnetic bar, and maintain the setup in an upright position by means of the plastic bar attached on the inner side of the bioreactor lid.
- Place the stainless-steel coupons or slides (see Table of Materials) on their polypropylene rods using the screwdriver, and insert them into the holes in the lid, without placing their alignment pins in the notches, to allow steam to escape during sterilization.
- Cover all the bioreactor vents with aluminum foil, and wrap the rest of the equipment, namely the tubing L/S 18 (ID = 7.9 mm) and L/S 16 (ID = 3.1 mm), the glass flow break, the container caps, the screwdrivers, the forceps, and 0.2 µm filters, with aluminum foil.
NOTE: Insert the silicon tubing (see Table of Materials) in the barb located on the inner surface of the medium container cap. - Autoclave the bioreactor set up in a dry cycle at 121 °C for 20 min.
- Perform biofilm formation in the bioreactor in batch mode (first step).
- In a BSC, connect one end of the tubing L/S 18 to the outlet spout of the bioreactor, and keep the other end wrapped in aluminum foil to preserve sterility.
- Remove one coupon or slide holder from the bioreactor lid and place it in a sterile 50 mL tube. Afterward, fill the beaker of the bioreactor with 340 mL of 300 mg/L TSB medium through the hole that was occupied by the rod using a 50 mL serological pipette.
- Inoculate the culture medium in the bioreactor with 1 mL of the bacterial solution (~108 cfu/mL of P. azotoformans) using a 5 mL pipette through the same orifice used previously, and then place the rod back into its original position. Position the rods that are already placed in the lid holes so that the pins fit into their respective notches.
- Place a 0.2 µm bacterial air purge filter at the end of the tube with the smallest diameter, which is located on the lid of the bioreactor. The other tube of the same diameter remains permanently plugged with a metal screw cap or a silicone plug that closes tightly.
- Place the bioreactor for 24 h over the heating plate set at 30 °C and stir at 130 rpm.
NOTE: For multispecies biofilm formation, use an equal volume of the different bacterial cultures to obtain a total volume of 1 mL for the inoculum.
- Perform biofilm formation in the bioreactor in continuous flow mode (second step).
- Place a carboy containing 18 L of sterile distilled water in the BSC, and add 2 L of 1,000 mg/L TSB culture medium to obtain a final concentration of 100 mg/L.
- Cover the container with its sterile cap, to which two tubes are connected. The first one is a silicone tube fixed on the internal face of the cap and is used to pump the medium. The second tube (L/S 16) is connected to the external port to allow the liquid to flow toward the bioreactor. Place a 0.2 µm filter into the second tube on the lid of the medium container.
- Connect this second tube to the peristaltic pump and join the other end to the glass flow break, which is then inserted into the larger tube on the bioreactor lid.
- Use another 20 L carboy to collect the effluent from the bioreactor. Attach the end of the tube connected to the bioreactor outlet spout to the cap of the waste container. Insert a 0.2 µm filter into the tube available on the lid of this container.
- Start the peristaltic pump at a flow rate of 11.3 mL/min, and leave the system running for 24 h.
NOTE: The flow rate (11.3 mL/min) of the culture medium or milk used during biofilm formation in the bioreactor was determined by dividing 340 mL (which corresponds to the volume of the liquid within the reactor) by the residence time of 30 min.
- Recover the bacterial biofilm.
- Turn off the peristaltic pump and stop stirring and heating the bioreactor.
- Carefully remove each rod from the bioreactor, and rinse the coupons or slides 3x in 40 mL of PBS to eliminate planktonic bacteria. After, release the coupons or slides into sterile 50 mL conical tubes containing 40 mL of PBS using an appropriate screwdriver. Vortex the tubes for 30 s, transfer them onto a rack placed in a sonicator bath, and sonicate the tubes at 40 kHz for 30 s (which requires 110 W of power). Repeat this operation 3x.
- Collect the 40 mL biofilm suspensions in sterile 50 mL conical tubes, and then rinse the coupons or slides with 2 mL of sterile PBS solution. Recover this rinsing liquid and add it to the already collected biofilm suspension.
- Enumerate the viable bacteria in the biofilm: With the obtained biofilm suspension, perform 10-fold serial dilutions, and then plate 100 µL of the 10−5 and 10−6 dilutions on TSA in triplicate. Incubate the plates at 30 °C for 24 h. Count the number of colonies present on the agar plates, and calculate the bacterial density on the coupons and slides (viable sessile bacteria) according to ASTM E2562-1714 using the following formula:
 Eq. (3)
Eq. (3)
where X is the number of colony-forming units (CFU), B is the volume plated (0.1 mL), V is the volume in which the biofilm is suspended (the stock solution), A is the surface of the coupon or slide covered by the biofilm, and D is the dilution factor.
- Clean and air-dry the parts of the bioreactor (see Table of Materials) following the manufacturer's instructions, and proceed to prepare the reactor as described below.
3. Quantitative evaluation of the efficacy of organic peroxyacids for eradicating biofilms
- Biofilm microtiter plates
- Add 200 µL of phosphate-buffered saline (1x PBS) into three wells of a 96-well microplate.
- Transfer the lid of the biofilm microplate, with biofilms that have formed on the pegs, to the 96-well microplate containing the PBS for 10 s in order to wash the biofilms and eliminate planktonic bacteria.
- Prepare the disinfectants at the required concentrations (e.g., 25 ppm, 50 ppm, 500 ppm, 1,000 ppm, 5,000 ppm, 10,000 ppm, and 25,000 ppm of active substance).
NOTE: All the dilutions are made aseptically using sterile distilled water. - Add 200 µL of each concentration of disinfectant into the wells of a new 96-well microtiter plate in triplicate. Transfer the lid of the biofilm microtiter plate onto this 96-well microtiter plate containing the disinfectants, and incubate the plate at room temperature for the desired exposure time.
- Add 200 µL of Dey-Engley neutralizing broth to the wells of a new 96-well microtiter plate. Transfer the lid of the biofilm microtiter plate on the 96-well microtiter plate containing the neutralizing broth. Seal the microtiter plate with parafilm, and place it in the bath sonicator at 40 kHz for 30 min.
- After 30 min, remove the microtiter plate from the sonicator and remove the parafilm. Transfer 100 µL from the first column of the 96-well plate containing the biofilms detached following sonication to the first row of a new 96-well microtiter plate.
- Add 180 µL of sterile 1x PBS to the wells of the new 96-well microplate (prepared in step 3.1.6), except for the first row. Transfer 20 µL of the biofilm solution from the first row to the wells in the second row containing 180 µL of 1x PBS (Row 2, dilution: 10−1). Then, transfer 20 µL of the liquid contained in the second row to the wells in the next row containing 180 µL of 1x PBS (Row 3, dilution: 10−2). Repeat the same procedure to obtain dilutions between 10−5 and 10−7.
- Inoculate 100 µL of the dilutions on TSA, and incubate the plates according to the parameters required for the growth of each bacterium.
- After incubation, count the cfus and calculate the log10 density for each peg and the log10 reduction at each disinfectant concentration with the following equations:
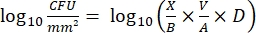 Eq. (4)
Eq. (4)
where X is the colony-forming units counted in the spot, B is the volume plated (0.01 mL), V is the well volume (0.20 mL), A is the peg surface area (46.63 mm2), and D is the dilution.
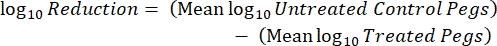 Eq. (5)
Eq. (5) - Repeat each experiment 3x on independent days.
NOTE: The "minimum concentration of disinfectant that eradicates the biofilm", or MBEC, corresponds to the lowest disinfectant concentration that shows no bacterial growth.
- Bioreactor
NOTE: The procedure of the ASTM standard E2871-1915 is followed to perform this test with P. azotoformans PFlA1.- Start by forming the biofilms on the coupons in the bioreactor, as described in step 2.2. Then, remove the rod that holds the coupons and rinse it inside a conical tube containing 30 mL of PBS.
- Drop each coupon into a sterile 50 mL conical tube using a screwdriver, and then add 4 mL of the appropriate organic peroxyacid solution or PBS for the control. Incubate for 5 min, and then add 36 mL of Dey-Engley neutralizing broth. Vortex for 30 s, and then sonicate at 40 kHz for 30 s using a sonicator bath. Repeat the process 3x to obtain the biofilm suspension.
- Similarly, rinse all the other rods and recover the biofilms from the coupons. Perform serial dilutions of the biofilm suspension and plate 0.1 mL on TSA medium. Incubate the plates at 30 °C for 24 h.
- After incubation, count the cfus, and then calculate the biofilm density for each coupon (Eq. 6), the mean log density (LD) for each set of three coupons from the same rod, including treated and control (Eq. 7), and the log reduction for the disinfectant (Eq. 8) using the following equations:
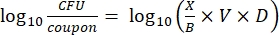 Eq. (6)
Eq. (6)
where X is the mean of the colony-forming units counted/coupon, B is the volume plated (0.1 mL), V is the volume of disinfectant or PBS plus neutralizer (40 mL), and D is the dilution.
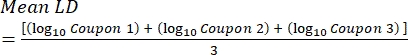 Eq. (7)
Eq. (7)
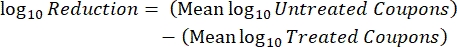 Eq. (8)
Eq. (8)
4. Qualitative evaluation of the efficacy of organic peroxyacids for eradicating biofilms
NOTE: After being treated with the disinfectants (step 3.1.1 to step 3.1.5), the P. azotoformans biofilms that formed on the pegs of the biofilm microtiter plate in the static method were prepared and analyzed by observation on scanning electron and confocal microscopes.
- Scanning electron microscopy (SEM)
- Add 200 µL of 1x PBS to three wells of a 96-well microplate. Transfer the lid from the biofilm microtiter plate (step 3.1.5) to the 96-well microplate containing PBS, and leave it at room temperature for 10 s to eliminate the Dey-Engley neutralizing broth.
- Remove the pegs from the biofilm microtiter plate using sterilized needle nose pliers. Place each peg into an empty vial under a hood, and add primary fixative (5% glutaraldehyde in 0.1 M Na cacodylate buffer pH 7.5) to each vial. Cap each vial and incubate them at 4 °C for 24 h.
- After incubation, decant the fixative with a pipette, and discard all the liquid waste in an appropriate container. Remove the caps of each vial and place them in a hood to air dry for 72 h.
- Mount the samples on aluminum stubs (see Table of Materials) by applying epoxy resin (see Table of Materials) to the flat upper surface of each stub. Then, carefully affix the pegs to the stubs with forceps.
- Metalize the samples with a EMS950x + 350s gold sputter (see Table of Materials) for 4 min at 2 x 10−1 bar argon pressure and 20 mA current. Perform appropriate grounding by painting the side of the peg unexposed to the gold with silver paint (see Table of Materials).
- Acquire images on a scanning electron microscope using the SEM control user interface version 6.28 (see Table of Materials). The acceleration voltage used in this study was 15 kV, and the magnifications were 300x and 2,000x.
- Confocal microscopy
- Add 200 µL of 1x PBS to three wells of a 96-well microtiter plate. Transfer the lid from the biofilm microplate to the 96-well plate containing the PBS, and leave it for 10 s to eliminate the Dey-Engley neutralizing broth.
- Prepare solutions of fluorescent stains by adding 3 µL of green fluorescent stain and 3 µL of red fluorescent stain (see Table of Materials) to 1 mL of sterile water.
- Add 200 µL of staining solution into one well of a 96-well microtiter plate. Transfer the lid from the biofilm microtiter plate to the 96-well plate containing the staining solution. Cover the biofilm microtiter plate with aluminum foil, and incubate the sample for 20-30 min at room temperature.
- Add 200 µL of sterilized water into the wells of a 96-well microplate. Then, transfer the lid from the biofilm microplate to the 96-well microplate containing the water, and leave it until observation.
- Visualize the biofilms formed on the pegs using a confocal laser scanning microscope (see Table of Materials) with a 63x/1.40 oil DIC objective. Acquire the images using the associated software (see Table of Materials). The fluorescence excitation wavelengths used for the green and red fluorescent stains were 482 nm and 490 nm, respectively.
NOTE: For the best results, cut and place the peg in a 60 mm Petri dish and fill the dish with sterile water.
Wyniki
The SEM analysis shows the presence of biofilms produced by P. azotoformans PFl1A on the biofilm microplate pegs (Figure 2A). A three-dimensional biofilm structure can be observed. The P. azotoformans PFl1A was previously identified as a strong biofilm producer (A570 > 1.5) using 96-well microtiter plates12.
In addition, the P. azotoformans PFl1A biofilm that formed on a stainless-steel slide using ...
Dyskusje
The MBEC assay (biofilm microplate assay) was the first method to be recognized as a standard biofilm eradication test by the ASTM17. Our study and others have shown that there are two critical steps when using this assay: the sonication step (time and power) and the disinfectant treatment time18. Stewart and Parker also suggested other parameters that could influence the outcome of the assay, such as the microbial species, biofilm age, surface area/volume ratio, etc.
Ujawnienia
The authors declare that they have no conflicts of interest.
Podziękowania
This research was supported by the Consortium de Recherche et Innovations en Bioprocédés Industriels au Québec (CRIBIQ)(2016-049-C22), Agropur, Groupe Sani Marc, and the Natural Sciences and Engineering Research Council of Canada (NSERC) (RDCPJ516460-17). We thank Teresa Paniconi for the critical review of the manuscript.
Materiały
| Name | Company | Catalog Number | Comments |
| 0.2 µm filters | Corning | 09-754-28 | diameter: 50 mm, PTFE- Membrane |
| 316 stainless-steel disc coupon | Biosurface Technologies Corporation | RD128-316 | |
| 316 stainless-steel slide coupon | Biosurface Technologies Corporation | CBR 2128-316 | |
| 96-microtiter plate | Corning | 07-200-89 | cell Culture-Treated, flat-Bottom Microplate |
| Acetic acid | Sigma Aldrich | 27225 | store at RT |
| Aluminium stubs | Electron Microscopy Science | 75830-10 | 32x5mm |
| Aqueous glutaraldehyde EM Grade 25% | Electron Microscopy Sciences | 16220 | store at -20 °C |
| AB204-S/FACT Analytical balance | Mettler Toledo | AB204-S | |
| Bacterial Vent Filter (0.45 µm) | Biosurface Technologies Corporation | BST 02915 | |
| BioDestroy | Groupe Sani Marc | 09-10215 | commercial peracetic acid-based disinfectant, store at RT |
| Carboy LDPE 20 L | Cole Parmer | 06031-52 | |
| CDC biofilm reactor | Biosurface Technologies Corporation | CRB 90 | bioreactor |
| Cerium (IV) sulphate | Thermo Scientific | 35650-K2 | store at RT |
| Confocal laser scanning microscope LSM 700 | Zeiss | LSM 700 | |
| Dey-Engley neutralizing broth | Millipore | D3435-500G | store at 4 °C |
| EMS950x + 350s gold sputter | Electron Microscopy Sciences | ||
| Epoxy resin | Electron Microscopy Sciences | 14121 | with BDMA |
| Ethyl alcohol 95%, USP | Greenfield global | P016EA95 | store at RT |
| Ferroin indicator solution | Sigma Aldrich | 318922-100ML | store at RT |
| Filling/venting cap | Cole Parmer | RK-06258-00 | |
| FilmTracer LIVE/DEAD Biofilm Viability Kit | Invitrogen | L10316 | fluorescent cell viability kit (SYTO 9: green fluorescent stain, Propidium iodide: red fluorescent stain), store at - 20 °C |
| Glass flow break | Biosurface Technologies Corporation | FB 50 | |
| Gold with silver paint | Electron Microscopy Sciences | 12684-15 | |
| Heating plate set | Biosurface Technologies Corporation | 110V Stir Plate | |
| Hex screwdriver | Biosurface Technologies Corporation | CBR 5497 | |
| Hydrogen peroxide | Sigma | 216763 | store at 4 °C |
| Inoculating loops | VWR | 12000-812 | sterile, 10 µl |
| Lactic acid | Laboratoire MAT | LU-0200 | store at RT |
| MASTERFLEX L/S 7557-04 W/ 7557-02 with EASY-LOAD II peristaltic pump and 77200-50 Head | Cole Parmer | 77200-60 | |
| MBEC (Minimum Biofilm Eradication Concentration) assay biofilm inoculator with a 96-well base | Innovotech | 19111 | Biofilm microtiter plate |
| Oxford agar base | Thermo Scientific | OXCM0856B | store at 4 °C |
| Plastic coupon holder | Biosurface Technologies Corporation | CBR 2203 | |
| Plastic slide holder rod | Biosurface Technologies Corporation | CBR 2203-GL | |
| Potassium iodide | Fisher Chemical | P410-500 | store at RT |
| Precision slotted screwdriver (1.5 mm x 40 mm) | Wiha | 26015 | |
| Propionic acid | Laboratoire MAT | PF-0221 | store at RT |
| Sartorius BCE822-1S Entris® II Basic Essential Toploading Balance | Cole Parmer | UZ-11976-3 | |
| Scanning electron microscope JSM-6360LV model | JEOL | JSM-6360LV | SEM and user control interface |
| Screw cap tube, 15 mL | Sarstedt | 62.554.205 | (LxØ): 120 x 17 mm, material: PP, conical base, transparent, HD-PE |
| Screw cap tube, 50 mL | Sarstedt | 62.547.205 | (LxØ): 114 x 28 mm, material: PP, conical base, transparent, HD-PE |
| Sodium Cacodylate Trihydrate | Electron Microscopy Sciences | 12300 | store at -20 °C |
| Sodium thiosulfate | Thermo Scientific | AC124270010 | store at RT |
| Sonication bath | Fisher | 15-336-122 | 5,7 L |
| Starch solution | Anachemia | AC8615 | store at RT |
| Sulfuric acid | Sigma Aldrich | 258105-500ML | store at RT |
| Tryptic soy agar | BD Bacto | DF0369-17-6 | store at RT |
| Tryptic soy broth | BD Bacto | DF0370-17-3 | store at RT |
| Tubing Masterflex L/S 16 25' | Cole Parmer | MFX0642416 | |
| Tubing Masterflex L/S 18 25' | Cole Parmer | MFX0642418 | |
| Tygon SPT-3350 silicon tubing | Saint-Gobain | ABW18NSF | IDx OD: 1/4 in.x 7/16 in. |
| Vortex | Cole Parmer | UZ-04724-00 | |
| Water bath | VWR | 89202-970 | |
| Zen software | Zeiss |
Odniesienia
- Canada's dairy industry at a glance. Canadian Dairy Information Centre Available from: https://agriculture.canada.ca/en/canadas-agriculture-sectors/animal-industry/canadian-dairy-information-centre/canadas-dairy-industry-glance (2017)
- Oliver, S. P., Jayarao, B. M., Almeida, R. A. Foodborne pathogens in milk and the dairy farm environment: food safety and public health implications. Foodborne Pathogens and Disease. 2 (2), 115-129 (2005).
- Fondation de technologie laitière du Québec. . Science et technologie du lait. 3rd edn. , (2018).
- Evanowski, R., et al. Short communication: Pseudomonas azotoformans causes gray discoloration in HTST fluid milk. Journal of dairy science. 100, 7906-7909 (2017).
- Bower, C. K., McGuire, J., Daeschel, M. A. The adhesion and detachment of bacteria and spores on food-contact surfaces. Trends in Food Science & Technology. 7 (5), 152-157 (1996).
- Gupta, S., Anand, S. Induction of pitting corrosion on stainless steel (grades 304 and 316) used in dairy industry by biofilms of common sporeformers. International Journal of Dairy Technology. 71 (2), 519-531 (2018).
- Marchand, S., et al. Biofilm formation in milk production and processing environments; Influence on milk quality and safety. Comprehensive Reviews in Food Science and Food Safety. 11 (2), 133-147 (2012).
- Silva, H. O., et al. Efficiency of different disinfectants on Bacillus cereus sensu stricto biofilms on stainless-steel surfaces in contact with milk. Frontiers in Microbiology. 9, 2934 (2018).
- Sedlak, D. L., von Gunten, U. Chemistry. The chlorine dilemma. Science. 331 (6013), 42-43 (2011).
- vander Veen, S., Abee, T. Mixed species biofilms of Listeria monocytogenes and Lactobacillus plantarum show enhanced resistance to benzalkonium chloride and peracetic acid. International Journal of Food Microbiology. 144 (3), 421-431 (2011).
- Saa Ibusquiza, P., Herrera, J. J., Cabo, M. L. Resistance to benzalkonium chloride, peracetic acid and nisin during formation of mature biofilms by Listeria monocytogenes. Food Microbiology. 28 (3), 418-425 (2011).
- Goetz, C., Larouche, J., Velez Aristizabal, M., Niboucha, N., Jean, J. Efficacy of organic peroxyacids for eliminating biofilm preformed by microorganisms isolated from dairy processing plants. Applied and Environmental Microbiology. 88 (4), 0188921 (2022).
- Vimont, A., Fliss, I., Jean, J. Study of the virucidal potential of organic peroxyacids against norovirus on food-contact surfaces. Food and Environmental Virology. 7 (1), 49-57 (2015).
- ASTM E2562-17. Standard Test Method for Quantification of Pseudomonas aeruginosa Biofilm Grown with High Shear and Continuous Flow using CDC Biofilm Reactor. ASTM International Available from: https://www.astm.org/e2562-17.html (2017)
- ASTM E2871-19. Standard Test Method for Evaluating Disinfectant Efficacy Against Pseudomonas aeruginosa Biofilm Grown in CDC Biofilm Reactor Using Single Tube Method. ASTM International Available from: https://www.astm.org/e2871-19.html (2019)
- Niboucha, N., et al. Comparative study of different sampling methods of biofilm formed on stainless-steel surfaces in a CDC biofilm reactor. Frontiers in Microbiology. 13, 892181 (2022).
- ASTM E2799-17. Standard Test Method for Testing Disinfectant Efficacy against Pseudomonas aeruginosa Biofilm using the MBEC Assay. ASTM International Available from: https://www.astm.org/e2799-17.html (2022)
- Parker, A. E., et al. Ruggedness and reproducibility of the MBEC biofilm disinfectant efficacy test. Journal of Microbiological Methods. 102, 55-64 (2014).
- Stewart, P. S., Parker, A. E. Measuring antimicrobial efficacy against biofilms: A meta-analysis. Antimicrobial Agents and Chemotherapy. 63 (5), 00020 (2019).
- Lindsay, D. K., Fouhy, K., Loh, M., Malakar, P. The CDC biofilm bioreactor is a suitable method to grow biofilms, and test their sanitiser susceptibilities, in the dairy context. International Dairy Journal. 126, 105264 (2022).
- Buckingham-Meyer, K., Goeres, D. M., Hamilton, M. A. Comparative evaluation of biofilm disinfectant efficacy tests. Journal of Microbiological Methods. 70 (2), 236-244 (2007).
- Goeres, D. M., et al. Statistical assessment of a laboratory method for growing biofilms. Microbiology (Reading). 151, 757-762 (2005).
- Williams, D. L., Woodbury, K. L., Haymond, B. S., Parker, A. E., Bloebaum, R. D. A modified CDC biofilm reactor to produce mature biofilms on the surface of peek membranes for an in vivo animal model application. Current Microbiology. 62 (6), 1657-1663 (2011).
- Pieranski, M. K., Rychlowski, M., Grinholc, M. Optimization of Streptococcus agalactiae biofilm culture in a continuous flow system for photoinactivation studies. Pathogens. 10 (9), 1212 (2021).
- Mendez, E., Walker, D. K., Vipham, J., Trinetta, V. The use of a CDC biofilm reactor to grow multi-strain Listeria monocytogenes biofilm. Food Microbiology. 92, 103592 (2020).
- Salgar-Chaparro, S. J., Lepkova, K., Pojtanabuntoeng, T., Darwin, A., Machuca, L. L. Nutrient level determines biofilm characteristics and subsequent impact on microbial corrosion and biocide effectiveness. Applied and Environmental Microbiology. 86 (7), 02885 (2020).
- Goeres, D. M., Simoes, M., Borges, A., Chaves Simoes, L., et al. Design and Fabrication of Biofilm Reactors. Recent Trends in Biofilm Science and Technology. , 71-88 (2020).
- Fjeld, C. S., Schüller, R. B. Biofilm formation during hexadecane degradation and the effects of flow field and shear stresses. Annual Transactions - The Nordic Rheology Society. 21, 341-346 (2013).
- Gilmore, B. F., Hamill, T. M., Jones, D. S., Gorman, S. P. Validation of the CDC biofilm reactor as a dynamic model for assessment of encrustation formation on urological device materials. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 93 (1), 128-140 (2010).
- Picioreanu, C., van Loosdrecht, M. C., Heijnen, J. J. Two-dimensional model of biofilm detachment caused by internal stress from liquid flow. Biotechnology and Bioengineering. 72 (2), 205-218 (2001).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone