Curvas de calibração
Visão Geral
Fonte: Laboratório da Dra.B. Jill Venton - Universidade da Virgínia
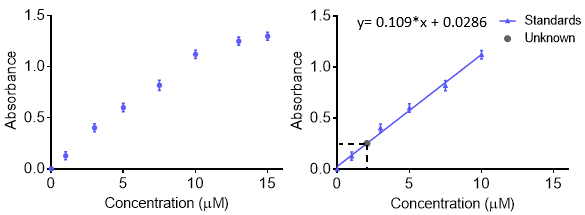
As curvas de calibração são usadas para entender a resposta instrumental a um analito e prever a concentração em uma amostra desconhecida. Geralmente, um conjunto de amostras padrão são feitas em várias concentrações com uma faixa que inclui o desconhecido de interesse e a resposta instrumental em cada concentração é registrada. Para obter mais precisão e entender o erro, a resposta em cada concentração pode ser repetida para que uma barra de erro seja obtida. Os dados são então adequados com uma função para que concentrações desconhecidas possam ser previstas. Normalmente a resposta é linear, no entanto, uma curva pode ser feita com outras funções, desde que a função seja conhecida. A curva de calibração pode ser usada para calcular o limite de detecção e limite de quantitação.
Ao fazer soluções para uma curva de calibração, cada solução pode ser feita separadamente. No entanto, isso pode levar muito material inicial e ser demorado. Outro método para fazer muitas concentrações diferentes de uma solução é usar diluições seriais. Com diluições seriais, uma amostra concentrada é diluída de forma stepwise para fazer concentrações mais baixas. A próxima amostra é feita a partir da diluição anterior, e o fator de diluição é muitas vezes mantido constante. A vantagem é que apenas uma solução inicial é necessária. A desvantagem é que quaisquer erros na criação de soluções — pipetação, massing, etc.— são propagados à medida que mais soluções são feitas. Assim, deve-se tomar cuidado ao fazer a solução inicial.
Procedimento
1. Fazendo os Padrões: Diluições seriais
- Faça uma solução concentrada de estoque do padrão. Normalmente, o composto é pesado com precisão e, em seguida, transferido quantitativamente para um frasco volumoso. Adicione algum solvente, misture para que a amostra se dissolva e, em seguida, encha a linha com o solvente adequado.
- Realizar diluições seriais. Pegue outro frasco volumoso e pipeta a quantidade de padrão necessária para a diluição, em seguida, encha a linha com solvente e misture. Uma diluição d
Aplicação e Resumo
As curvas de calibração são usadas em muitos campos de química analítica, bioquímica e química farmacêutica. É comum usá-los com medições de espectroscopia, cromatografia e eletroquímica. Uma curva de calibração pode ser usada para entender a concentração de um poluente ambiental em uma amostra de solo. Pode ser usado para determinar a concentração de um neurotransmissor em uma amostra de fluido cerebral, vitamina em amostras farmacêuticas ou cafeína em alimentos. Assim, as curvas de calibração sã...
Pular para...
Vídeos desta coleção:

Now Playing
Curvas de calibração
Analytical Chemistry
798.5K Visualizações

Preparação da Amostra para Caracterização Analítica
Analytical Chemistry
85.2K Visualizações

Normas Internas
Analytical Chemistry
205.3K Visualizações

Método de adição de padrão
Analytical Chemistry
320.7K Visualizações

Espectroscopia ultravioleta-visível (UV-Vis)
Analytical Chemistry
625.1K Visualizações

Espectroscopia raman para análise química
Analytical Chemistry
51.4K Visualizações

Fluorescência de raios X (XRF)
Analytical Chemistry
25.9K Visualizações

Cromatografia gasosa (GC) com detecção por ionização em chama
Analytical Chemistry
283.0K Visualizações

Cromatografia Líquida de Alta Eficiência (HPLC)
Analytical Chemistry
385.8K Visualizações

Cromatografia de troca iônica
Analytical Chemistry
265.0K Visualizações

Eletroforese Capilar (CE)
Analytical Chemistry
94.4K Visualizações

Introdução à Espectrometria de Massa
Analytical Chemistry
112.8K Visualizações

Microscopia Eletrônica de Varredura (MEV)
Analytical Chemistry
87.6K Visualizações

Medições eletroquímicas de catalisadores suportados usando um potenciostato/galvanostato
Analytical Chemistry
51.8K Visualizações

Voltametria Cíclica (CV)
Analytical Chemistry
125.9K Visualizações
Copyright © 2025 MyJoVE Corporation. Todos os direitos reservados