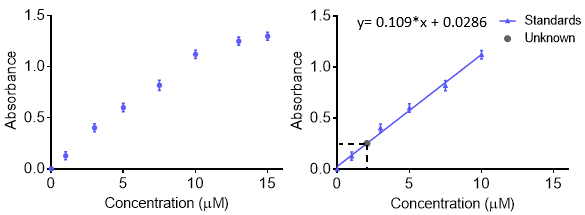
校准曲线
Overview
资料来源: 实验室的博士 B.吉尔 Venton-弗吉尼亚大学
校准曲线用于了解仪器响应分析物和预测未知样品的浓度。一般来说,一套标准样品在不同浓度范围不包括未知的兴趣和记录在每个浓度的仪器响应。要更准确,理解错误,可以重复在每个浓度的响应,因此获得一条误差线。数据然后符合一个函数,可以预测未知的浓度。通常的反应是线性,但是,曲线可与其他函数,只要功能而闻名。校准曲线可以用于计算检测限和定量限。
在校准曲线的解决方案时,可以分别进行每个解决方案。然而,这可以采取大量的起始原料和耗时。许多不同溶液浓度的另一种方法是使用串行稀释。随着串行的稀释度,以逐步的方式,使较低的浓度稀释浓缩的样品。下一个示例由以前的稀释,且稀释因子经常保持恒定。优点是只有一个初始解需要。缺点是,解决方案制作中的任何错误 — — 吹打,集结,等等 — — 得到传播作出更多的解决方案。因此,必须小心进行最初的解决方案时。
Procedure
1.使标准: 系列稀释
- 让股票浓缩液的标准。通常情况下,该化合物是准确称出并定量地转移到容量瓶内。添加一些溶剂、 混合使样品溶解,然后填充到行用适当的溶剂。
- 执行系列稀释。采取另一容量瓶和移液器的标准所需的稀释量然后填充到行用溶剂和混合。十倍稀释通常做,因此为 10 毫升的容量瓶,加 1 毫升的以前的稀释。
- 继续根据需要为更多的稀释,用移液器从以前液稀释,使下一个样品。好的校准曲线,需要至少 5 浓度。
2.运行样品校准曲线和未知
- 紫外-可见分光光度法测定,以确定所需的校准曲线的仪器响应运行这些示例。
- 采取了阅读与第一个示例。它是一个好的主意,要运行这些示例以随机的顺序 (即不最高到最低或最低到最高),以防有任何系统性的错误。为了得到噪声估计,重复对任何给定的样本阅读 3 — — 5 倍。
- 运行附加的标准样品,重复测量得到的噪声估计每个样品。记录的数据,后来使情节。
- 运行未知的样品。用作对运行标准尽可能类
Application and Summary
跳至...
此集合中的视频:

Now Playing
校准曲线
Analytical Chemistry
794.7K Views

分析制备的样品制备
Analytical Chemistry
84.2K Views

内部标准
Analytical Chemistry
204.3K Views

标准加入的方法
Analytical Chemistry
319.4K Views

(紫外-可见) 的紫外-可见光谱法
Analytical Chemistry
621.7K Views

拉曼光谱化学分析
Analytical Chemistry
51.0K Views

X 射线荧光光谱 (XRF)
Analytical Chemistry
25.3K Views

气相色谱 (GC) 与火焰电离检测
Analytical Chemistry
281.1K Views

高性能液相色谱法 (HPLC)
Analytical Chemistry
383.1K Views

离子交换色谱法
Analytical Chemistry
263.9K Views

毛细管电泳 (CE)
Analytical Chemistry
93.3K Views

质谱分析法导论
Analytical Chemistry
111.9K Views

扫描电子显微镜 (SEM)
Analytical Chemistry
86.8K Views

负载型催化剂,用恒电位仪/结合电化学测量
Analytical Chemistry
51.3K Views

循环伏安法 (CV)
Analytical Chemistry
124.3K Views
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。