Method Article
Grossing of Non-neoplastic Globes, Including Fetal Eyes
In This Article
Summary
Thorough grossing and standardized sectioning of non-neoplastic globes are important for obtaining high-quality reproducible histopathologic sections. Guidelines for a standardized grossing of adult and fetal non-neoplastic globes are provided herein. Different options for globe sectioning are demonstrated and discussed, including criteria for case-based decision-making.
Abstract
Grossing and sectioning of fetal and adult globes are critical steps in the histopathologic evaluation of ocular diseases. Proper handling of the globe is essential for accurate diagnosis of conditions such as tumors and non-neoplastic changes, including trauma, infections, and previous surgical procedures. Orientation of the globe is followed by external examination, documenting characteristics including size, shape, and any visible lesion or abnormality. Specific attention must be paid to the presence of tumors, infectious or degenerative changes, as well as signs of trauma or prior surgery. Measurements of the globe and associated structures, including the cornea and optic nerve, and -- in fetal eyes -- anatomical and timely development are recorded. Transillumination is essential to detect a shadow, which may be caused by a mass or hemorrhage as well as increased light transmission in areas of atrophy, such as coloboma.
Fixation of the globe is routinely performed in 4% paraformaldehyde to preserve the tissue. After fixation for 24 h, the globe is sectioned systematically to examine intraocular structures, such as the anterior chamber, lens status, uvea, retina, vitreous, and optic nerve. The adult globe is usually trisected along the horizontal or -- depending on the clinical indications - vertical/oblique plane to create a pupil-optic nerve (PO) section. Additional sections of the calottes may be taken to evaluate areas of interest, such as suspicious masses or areas of atrophy. For research purposes, various fixation and sectioning protocols may be considered.
Each gross section is carefully inspected, documented, and processed for microscopic examination to ensure that any pathologic finding is adequately sampled. Accurate grossing and sectioning are critical for correlating clinical and histologic findings, facilitating diagnosis, guiding treatment decisions, and ultimately improving patient outcomes. Standardization of grossing and sectioning ensures a comprehensive evaluation of the eye globe and contributes to the advancement of ophthalmology.
Introduction
Gross examination and sectioning of eye globes are essential procedures in ophthalmic pathology, providing valuable insights into various ocular diseases and wound healing following surgery1. The eye, as a highly specialized organ, is prone to a wide range of pathophysiologic and/or pathologic changes, including tumors (e.g., uveal melanoma, retinoblastoma), inflammatory processes (e.g., uveitis, endophthalmitis), and neurodegenerative diseases (e.g., glaucoma, age-related macular degeneration)2,3,4. Wound healing following surgical procedures can also result in vision-threatening complications.
Adult eyes which are evaluated in an ophthalmic pathology laboratory are usually enucleated for clinical reasons, such as a tumor or a blind, painful eye. In most countries, the investigation of donor eyes from autopsies is restricted to research projects. In contrast, fetal and neonatal eyes are usually autopsy specimens (except for eyes with a clinical suspicion of an intraocular tumor)5,6. Thus, fetal and neonatal eyes are investigated in relation to findings from other disciplines, such as pediatric pathology or as part of a forensic autopsy7. For both fetal and adult eyes, knowledge of the patient's history before grossing is critical to understanding the indication of the histopathologic analysis, which has a significant impact on the way the eye is (photo-) documented and sectioned1,3.
However, accurate gross examination, sectioning, and documentation are not only critical for the histopathological evaluation, which contributes to the diagnosis, prognosis, and management of ocular diseases, but also for forensic cases (e.g., abusive head trauma or medicolegal aspects)8,9. During grossing, a thorough external examination of the globe is conducted under a dissecting microscope or a discarded ophthalmic surgery microscope, focusing on features such as size, shape, and surface characteristics (Supplemental Figure S1). Measurements of the entire globe (anterior-posterior, horizontal, and vertical), the cornea, and the optic nerve should be carefully performed by a caliper ruler and documented (Figure 1). The oblique muscles and the horizontal ciliary artery are helpful in orientating the globe. Remaining sutures can be indicative of a surgical procedure and need not be removed during grossing. However, some surgeons place a suture in one the rectus muscles during enucleation which may be helpful not only for removal of the globe but also for orientation (if the localization is reported). Precise documentation of any lesion, discoloration, or other abnormality is crucial, as these findings can provide early clues to the underlying pathology. Transillumination is mandatory for detecting intraocular tumors or developmental defects such as colobomas and identifying a site of a previous surgical procedure.
Sectioning of the globe is typically performed after fixation in 4% paraformaldehyde (4% PFA) or 10% formalin to preserve the tissue architecture. Routine fixation with 4% PFA is performed for 24 h. For electron microscopy, alternative fixatives may be used for research specimens, for example, Karnovsky's fixative (including glutaraldehyde) or 2.5% glutaraldehyde. An incision or injection of fixative is not recommended as it causes unnecessary artifacts. However, for research purposes, the globe may be opened to have better penetration of PFA, in particular, for fixation of the retina, but this is not advisable for routine specimens. Regarding donor eyes, they may be grossed in total or after removal of a sclero-corneal button for an eye bank.
The choice of the sectioning plane -- horizontal, vertical, or oblique -- depends on the clinical suspicion and the anatomical structures of interest, including sites of previous surgery (e.g., trabeculectomy for glaucoma, usually at the 12 o'clock position)10. Routinely, a globe is sectioned at the horizontal meridian to obtain a pupillary-optic nerve section (PO section) with the cornea, the pupil, the lens, the macula, and the optic nerve on one section. A PO section can also be achieved if a vertical or oblique section is performed. However, in this scenario, the macula will not appear on the same section as the optic nerve. The PO section is the gold standard for histopathological examination.
Following grossing and sectioning, the intraocular structures are thoroughly inspected, photodocumented, and described (Figure 1). Each ocular structure (cornea, lens status, iris, ciliary body, choroid, retina, vitreous body, optic nerve) should be examined separately. Whenever possible, a cross-section of the optic nerve should be obtained and submitted separately. All this examination provides the foundation for at least a macroscopic-histologic correlation if not a clinico-pathologic correlation.
Therefore, an accurate grossing and sectioning process is highly relevant in clinical practice because enucleated globes are submitted for histopathological analysis following severe trauma, suspected malignancies, or treatment-resistant infections. A standardized macroscopic examination protocol will enable ophthalmic pathologists to retrace the history of a globe to provide the most accurate histopathologic diagnoses with relevance not only for the patient's fellow eye but also for future patients. The following protocol describes and documents the handling of (non-neoplastic) fetal and adult eyes for routine clinical diagnosis, forensic investigations, and research approaches.
Protocol
Be sure to adhere to the safety instructions when handling formalin-fixed specimens as it is skin-irritating, carcinogenic, and embryotoxic11,12,13.
1. Orientation
- Identify the four rectus muscles (Figure 2A).
- Identify the superior oblique muscle by its tendinous appearance (Figure 2B). It inserts into the sclera on the posterotemporal surface of the globe in close proximity to the insertion of the superior rectus muscle.
- Identify the inferior oblique muscle. It inserts temporal inferiorly into the sclera between the lateral rectus muscle and the optic nerve (Figure 2C).
- Identify the long posterior ciliary artery (Figure 2B).
NOTE: In fetal eyes, the long posterior ciliary artery is critical for identifying the horizontal meridian since the muscles are not as prominent as in adult eyes7. - Determine the laterality of the globe accurately based on steps 1.1.1-1.1.3 (right eye versus left eye).
2. Grossing
- Measure the anterior-posterior diameter (AP diameter) as well as the horizontal and vertical diameter in mm (Figure 3A,B).
NOTE: For fetal eyes, the AP diameter must be compared to a nomogram to evaluate for microphthalmia7. - Describe any abnormalities of the external aspect of the globe including-but not limited to-scars, sutures, medical foreign material (e.g., encircling band for retinal detachment or glaucoma drainage device), foreign bodies, deformation (Figure 4A-D).
- Measure the cornea in its horizontal and vertical diameter in mm (Figure 3A).
- Describe the corneal aspect including-but not limited to-transparency, scars, sign of keratoplasty, sutures, vascularization (Figure 3A).
- Describe the iris (if visible) with regard to color, pupil configuration, and possible abnormalities. Use transillumination to evaluate iris defects (related to trauma or congenital abnormalities).
- Measure the optic nerve for length and abnormalities (e.g., hemorrhage in optic nerve sheaths) (Figure 3B).
- If possible, make a cross section through the optic nerve and submit it separately.
- Transilluminate the globe using an LED pipe with special emphasis for iris defects and a scleral shadow which might result from a tumor, hemorrhage, or foreign material (Figure 3C,D).
- Perform photo documentation of the globe.
3. Section of routine specimens
- Place the globe cornea down (Figure 5A).
- Mark the horizontal meridian or the section plane with a pen (Figure 5A-C) so that the section is close to the optic nerve and goes through the peripheral cornea. This also applies if the section is vertical or sagittal.
NOTE: Do not cut through areas of interest, instead cut close to it-otherwise, they may not be covered on the histologic section as the tissue shrinks through further processing. - Section carefully with a microtome blade along the marked line, holding the globe with the other fellow hand.
NOTE: Iatrogenic material, such as an encircling band, need not be removed (Figure 4). - Remove the calotte and inspect carefully for abnormalities (Figure 5D). Store in formalin or submit for further histological processing. If the globe is filled with silicon oil (used as tamponade) during retinal detachment surgery, take care of equipment since it is quite sticky.
- For uveal melanoma, remove the vortex vein; in non-tumorous eyes, this is not routinely done.
- Inspect the internal ocular structures: anterior chamber; lens status (phakic or pseudophakic, aphakic, cataract, secondary cataract); iris; ciliary body; choroid; central retina; peripheral retina; optic nerve (e.g., atrophy); sclera (may be thickened in phthisical eyes or thinned in trauma or staphyloma).
- Remove the other calotte to get a proper PO section (Figure 5E and Figure 6A); place the opened globe carefully with the opening downwards.
- Hold the globe with one hand and section it in a parallel direction (but above the optic nerve and again ideally through the peripheral cornea). The globe is less stable once opened.
- Remove the calotte and inspect carefully for abnormalities. Store in formalin or submit for further histological processing (Figure 6).
- Perform photo documentation of the PO section and submit it for further histological processing.
4. Section of fetal specimens
NOTE: These are only adjustments to the main protocol described in sections 1 (orientation), 2 (grossing), and 3 (section of routine specimens).
- Position the globe with the cornea down.
- Identify the long posterior ciliary artery by gently compressing the globe (Figure 7).
- Dissect the globe horizontally close to the optic nerve, putting slight pressure on the microtome blade. Ensure that the periphery of the cornea is also within the section.
NOTE: Trisection is not recommended due to the small size of the fetal globe. - Evaluate the intraocular structures thoroughly and photo document it as described in step 3.6.
NOTE: Special attention should be paid to fetal structures (e.g., fetal vasculature) or development abnormalities (e.g., coloboma). - Submit the main part of the globe for further processing.
- Archive the remaining calotte in formalin or submit for further processing.
5. Section of forensic specimens
- Be familiar with the forensic question before sectioning. In forensic pathology, prepare to analyze parts of the globe toxicologically (vitreous) or use them to estimate the time since death (cornea)2,14.
- For abusive head trauma (AHT), perform a horizontal dissection followed by thorough inspection and photo documentation of retinal hemorrhages and optic nerve sheath hemorrhages. Perform an iron stain (e.g., Perl's Prussian blue stain) to evaluate signs of already degraded hemorrhages.
NOTE: A more detailed protocol is provided by Gilliland et al. who recommend removing and analyzing the eyes and their orbital contents15.
There are many other conditions leading to retinal hemorrhages in a newborn apart from AHT7,16,17,18,19,20. - For surgery-related questions (e.g., endophthalmitis4), carry out horizontal dissection of the globe and evaluation as described in section 3. For eyes with a history of trabeculectomy, perform a vertical section close to the bleb-site (which may be an entrance for bacteria).
- Foreign bodies: Measure and photograph the object and its location in situ or the void space where the object was lodged (metal foreign bodies require removal before cutting as they cannot be sectioned). Describe the color, shape, and consistency. Keep the foreign body stored as it may be reinvestigated for legal purposes.
6. Alternative approaches
NOTE: An alternative approach can be used if the retina needs to be investigated before sectioning10,21,22. This approach is similar to donor globes where sclerocorneal tissue has been removed for an eye bank.
- Use a scalpel and cut circumferentially within the pars plana (Figure 8A).
- Remove the anterior segment (cornea, scleral rim, lens), bisect, and submit for histopathological routine evaluation (Figure 8B).
- Visualize the retina and image it multimodally (before and/or after fixation) (Figure 8B).
- Remove both calottes for further routine processing. As the anterior segment has been already removed, the remaining globe is instable; hence, stabilize it securely with one hand while removing the calotte as described in steps 3.1-3.4.
- Evaluate the intraocular structures thoroughly and photograph as described in step 3.6.
- Remove the second calotte as described in steps 3.7-3.10 (Figure 8D).
NOTE: For research-only specimens, other approaches are possible21.
7. Further processing
- After routine processing and paraffin embedding, prepare step sections of 5 µm thickness until the optic nerve and macula are on the section.
- Individually determine the distance between the step sections.
NOTE: This distance between the step sections is routinely ~250 µm in our ophthalmic pathology laboratory. - Prepare serial sections for research globes. If immunohistochemical stains are needed, mount the sections on coated slides.
Results
After orientation, it should be feasible to determine the laterality for adult eyes. However, this is often impossible for fetal eyes. Laterality is important to confirm that the correct eye has been removed and that it matches the report. The measurements before sectioning are documented. In particular, for fetal eyes a nomogram is helpful to rule out anomalies of ocular size such as microphthalmia.
Based on the patient's history and transillumination, sectioning is planned. The globe is usually sectioned horizontally, which requires proper identification of the horizontal plane as described above. If there are specific areas of interest (e.g., coloboma, unsuspected lesions identified on transillumination, or surgical sites such as trabeculectomy), the sectioning is adapted respectively. The target result of sectioning is a proper PO section (Figure 5 and Figure 6).
An awkwardly sectioned globe impairs a precise clinicopathologic correlation and interpretation of the histologic findings with a potential impact on patient follow-up. For instance, one of the most threatening diagnoses to consider is sympathetic ophthalmia23 a bilateral, chronic granulomatous inflammation of the uvea potentially leading to blindness of the remaining fellow eye (sympathizing eye). This entity needs to be ruled out in every globe and, in particular, in blind, painful eyes. As this is critical for preserving the remaining eye, representative sections of the globe are mandatory for evaluation, preferably as PO sections.
In the event that a cut is erroneously made through the entire cornea instead of sectioning the periphery, submit the calotte with cornea and anterior segment and the failed PO section. The second calotte needs to be removed as described above to have the globe fit into the cassette. Notably, phthisical eyes can exhibit osseous metaplasia of the retinal pigment epithelium, making it nearly impossible to section the globe. Decalcify the globe in 5% nitric acid for 24 h followed by 5% lithium or sodium sulfate solution for 24 h, store in tap water for 24-48 h and try again to cut the globe. Repeat decalcification until the globe can be sectioned. Lastly, photodocumentation of every globe should be performed. This is not only for forensic reasons but also helpful if unexpected histologic findings occur.
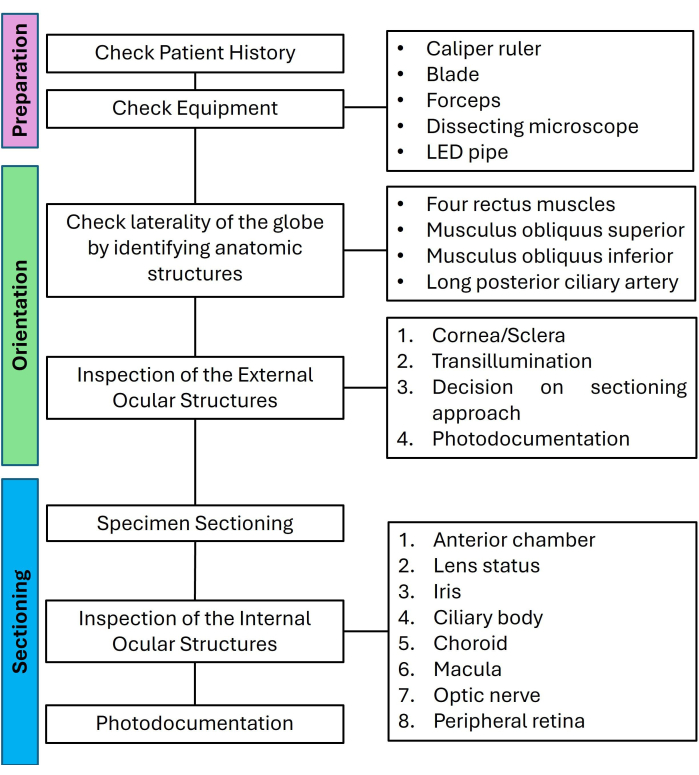
Figure 1: Flow chart for preparation, orientation, and sectioning of non-tumor eyes listing the relevant steps in a checklist-like approach. Abbreviation: LED = light-emitting diode. Please click here to view a larger version of this figure.
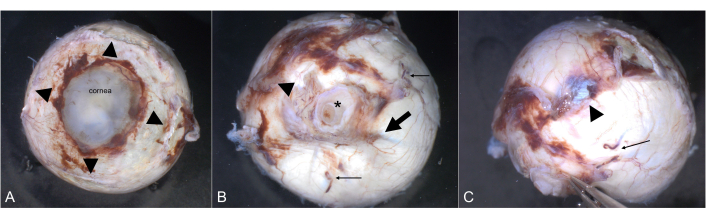
Figure 2: Orientation. The cornea is clearly visible from an anterior view. (A) The insertions of the four rectus muscles are highlighted by arrowheads. (B) The optic nerve (asterisk) is visible from a posterior view. The long ciliary artery (arrow) marks the horizontal meridian. The typically fleshy insertion of the inferior oblique muscle (arrowhead) is roughly temporal. Two of the vortex veins are highlighted by thin arrows. (C) The tendinous insertion of the superior oblique muscle (arrowhead) is superiorly between the superior rectus muscle and the optic nerve. A vortex vein is marked by a thin arrow. Please click here to view a larger version of this figure.
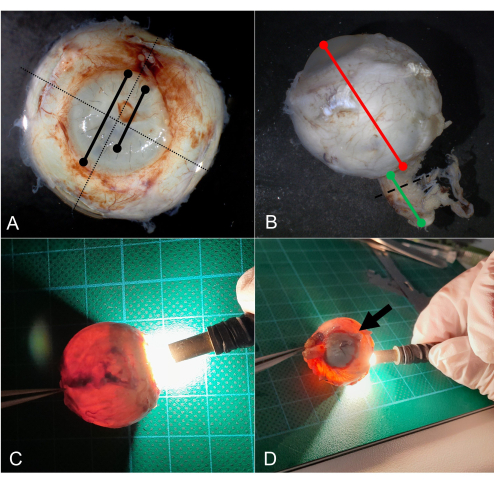
Figure 3: Grossing. (A) Measurement of the horizontal and vertical diameter in mm (dotted line). Measurement of the corneal diameter, and for example, the diameter of the keratoplasty. (B) Measure the anterior-posterior diameter in mm (red line) and the length of the optic nerve (green line). The optic nerve is cross-sectioned (dotted line) and separately submitted for further processing. (C) Regular transillumination of a globe by an LED light. (D) On transillumination, a shadow is seen caused by a choroidal tumor (arrow). Abbreviation: LED = light-emitting diode. Please click here to view a larger version of this figure.
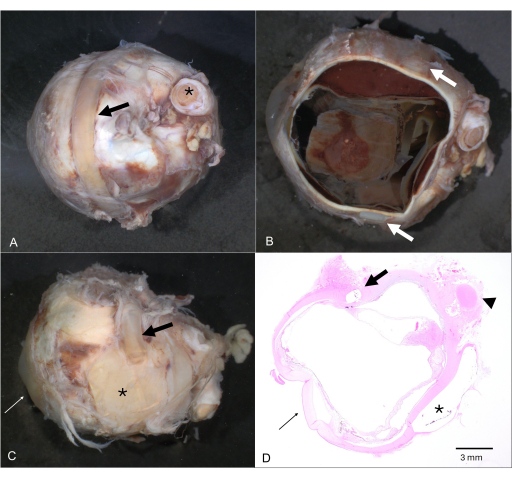
Figure 4: Extraocular foreign material. (A) Globe with an encircling band/cerclage (arrow; optic nerve is marked by an asterisk). (B) After horizontal dissection, the encircling band is still attached to the globe (arrows). (C) Multiple external foreign material applications for historic retinal detachment surgery consisting of plates (asterisk) and several scleral buckles (arrow). (D) The corresponding Hematoxylin & Eosin section shows the intrascleral location of a buckle (arrow) as well as the negative image of an extrascleral plate (asterisk). The cornea is marked in C and D by a thin arrow and the optic nerve by an arrowhead in D. Please click here to view a larger version of this figure.
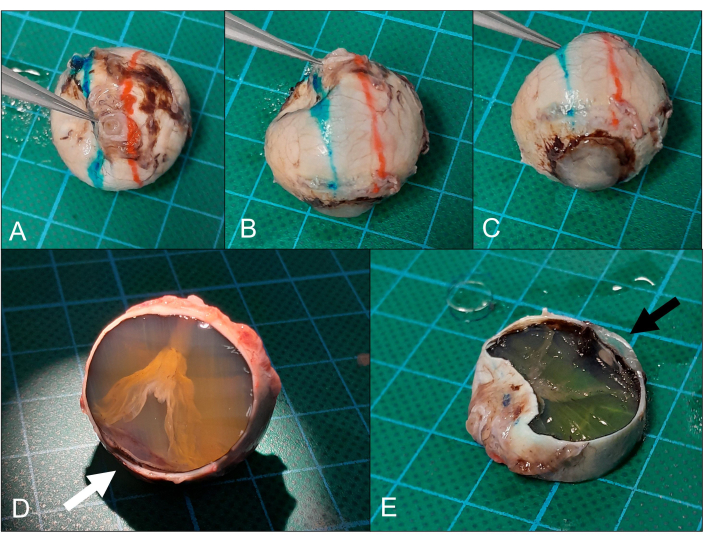
Figure 5: Sectioning. (A) The globe is placed cornea down (same eye as in Figure 2) and (B) the superior (red) and inferior (blue) section planes are marked. (C) The section plane goes ideally through the peripheral cornea/limbal area. (D) After the removal of the superior calotte, a funnel-shaped retinal detachment can be seen. The section is only close to the peripheral cornea (arrow) but this is also sufficient for analysis. (E) Removal of the inferior calotte (note the peripheral cornea on the section, arrow) yields the intended pupil-optic nerve section. Please click here to view a larger version of this figure.
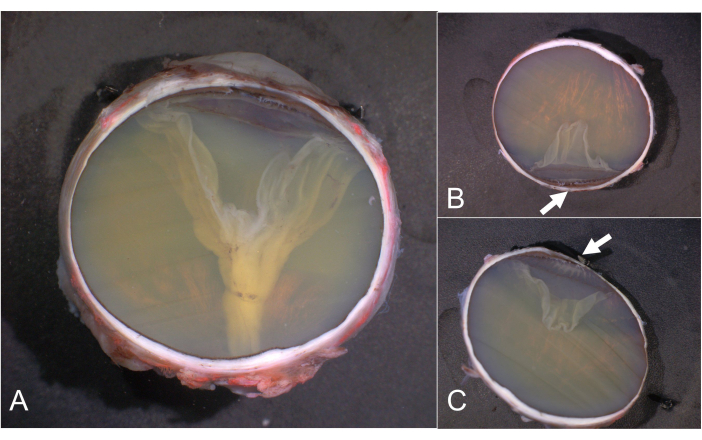
Figure 6: PO sections and calottes. (A) PO section, (B) superior, and (C) inferior calotte. The pars plicata of the ciliary body is highlighted by arrows in B and C. Calottes without clinically relevant details do not have to be further processed and are archived instead. Abbreviation: PO = pupil-optic nerve. Please click here to view a larger version of this figure.
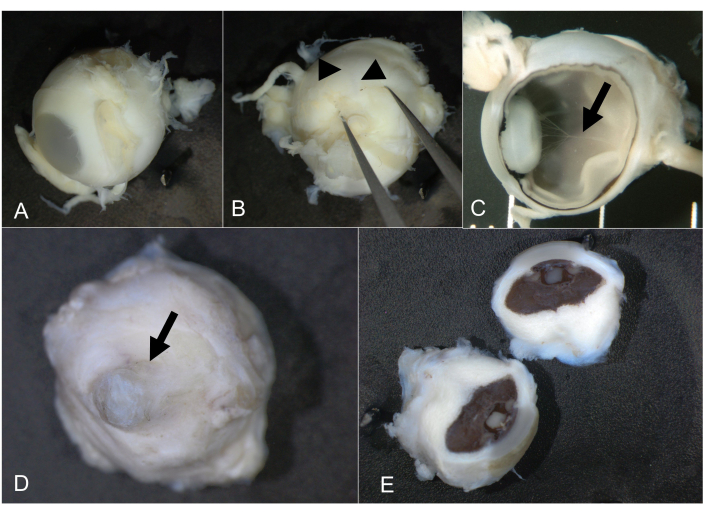
Figure 7: Fetal and microphthalmic eyes. (A) Regular fetal eye, 21 weeks of gestation and 10 mm in AP diameter. (B) Pushing gently with forceps reveals the long posterior ciliary artery (arrowheads), which indicates the horizontal meridian. (C) After bisection, the intraocular structures can be inspected including the hyaloid artery (arrow). (D) Microphthalmic eye from an adult. The cornea is highlighted by an arrow. (E) The globes were bisected; the long posterior ciliary artery could not be identified. Abbreviation: AP = anterioposterior. Please click here to view a larger version of this figure.
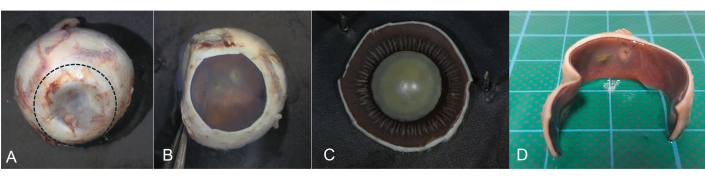
Figure 8: Coronal incision. A coronal incision is helpful if postmortem imaging of the fundus is intended. During transillumination, the pars plana of the ciliary body can be identified as a circular band-like structure. (A) Using a scalpel, a circular incision is made in the pars plana area (dotted line) and the anterior segment is removed. (B) Retina and optic nerve can be documented and imaged. (C) The anterior segment is then investigated separately. (D) Horizontal section with removed anterior segment. Please click here to view a larger version of this figure.
Supplemental Figure S1:Dissecting microscope unit. Work unit with a discarded ophthalmic surgery microscope. (A) An LED pipe (arrow) and the equipment needed for grossing and sectioning. Materials needed are (B) ruler, caliper ruler, forceps, and (C) microtome/razor blades. For sectioning of adult globes, microtome blades (right) are recommended; for fetal eyes or other ocular specimens, razor blades (left) are sufficient. (D) Dissecting microscope with a connected camera to document the specimen. Please click here to download this File.
Discussion
This protocol aims to standardize gross examination of non-neoplastic eye globes. Grossing guidelines in the literature focus mainly on eyes with an intraocular tumor24,25. The respective sectioning protocols are found at the website of the American Academy of Ophthalmology26, at various university websites, at the website of the College of American Pathologists, as well as in ophthalmic pathology textbooks7,10. There are also specific protocols for research purposes, for example, light sheet fluorescence for the ocular vasculature27,28,29.
To the best of our knowledge, there is no published directive for systematic grossing of enucleated non-tumoral globes. Notably, our guideline aims to enhance the evaluation of enucleation specimens, which differs from tumor eyes in that the main focus of investigation may not be predetermined. Moreover, the non-tumor eyes usually have a long ocular history, including surgery, with manifold intraocular changes elucidating the pathophysiology of ocular diseases and wound healing. Evisceration -- the intraocular structures are removed while the sclera and optic nerve remain in the patient -- does not allow for a precise location of ocular findings.
In cases where enucleation is required for indications other than a tumor, all other available treatment options have failed and patients typically report a blind, painful eye due to secondary glaucoma (e.g., following proliferative diabetic retinopathy or central retinal vein occlusion), severe ocular trauma, uncontrolled perforating corneal diseases, or frustrating retinal detachment surgery (which has become rare due to improved retinal surgery techniques). As ophthalmology is a highly specialized surgical discipline, there are many options to preserve the patients' vision or at least keep the eye in a painless state and the above-mentioned reasons for enucleation are fortunately rare. Many patients undergoing enucleation have, therefore, a history of multiple surgical interventions such as keratoplasty, pressure-lowering, or retinal surgery. Tracing the patient's history, which is not always mentioned in detail on the pathology request form, is critical for the decision on how to section the eye.
A comprehensive examination of enucleation specimens improves patient care by better understanding the severe eye conditions that can lead to this ultimate surgical procedure. Adequate grossing also enhances the correlation between multimodal imaging and histological findings, as described by Eagle21. Moreover, the gross examination of globes has great educational value, as it helps students and residents better understand the complexity and extent of various ocular diseases and the implications of surgical interventions.
For fetal eyes, the standardized examination helps with the exact morphological diagnosis of eye malformations. Together with the other findings obtained during fetal autopsy, it often allows for an exact classification of a syndrome and thus improved genetic counseling for parents regarding the risk of recurrence in subsequent pregnancies.
Correct positioning of the specimen is a critical step in applying this protocol. An error in this phase of examination can compromise the description and localization of the findings, producing an incorrect report. One limiting key factor of this method is the preservation of the globe. A specimen left without formalin for many hours may exhibit signs of autolysis, which may compromise histopathological evaluation, including immunohistochemical staining1. Further, architectural distortions, which are often found in phthisical eyes, might make it difficult to correctly position the globe and make a precise and symmetrical incision. To overcome this, look for the key anatomic structures.
In conclusion, we propose a systematic protocol for a comprehensive examination of enucleated eyes, aiming at improving patient care through enhanced diagnostic accuracy and at providing educational opportunities. Our approach focuses on the proper identification of anatomical structures and spatial orientation of the specimen, ensuring correct assessment and documentation of pathological alterations. The standardization of gross examination of non-neoplastic globes should be implemented in pathology laboratories to improve descriptive and diagnostic ophthalmic pathology for clinical and educational purposes and support future studies on clinicopathologic correlations.
Disclosures
Martina C. Herwig-Carl is the co-author of the book "Fetal and Neonatal Eye Pathology", Springer 2020; Secretary of the International Society of Ophthalmic Pathology (ISOP); Member of the section committee "ophthalmic pathology" within the German Ophthalmological Society (DOG); Member of the "Annual Meeting Program Committee" of ARVO for the section "Anatomy Pathology (AP)". Frank G. Holz: Acucela (C,F,R), Alcon (C,F,R), Alexion, Apellis (C,R), Allergan/AbbVie company (F,R), Bayer (C,F,R), Boehringer Ingelheim (C), CenterVue (F), Ellex (R), Genentech/Roche (C,F,R), Geuder (C), Grayburg Vision (C,R), Kanghong (C,F), Heidelberg Engineering (C,F,R), Lin BioScience (C,R), NightStarX (F), Novartis (C,F,R), Optos (F), Oxurion (C,R), Pixium Vision (C,F,R), Stealth Biotherapeutics (C,R), Zeiss (F,R). The other authors have no conflicts of interest to declare. [C: consultant; F: financial support; R: recipient]
Acknowledgements
This work was supported by the Open Access Publication Fund of the University of Bonn.
Materials
| Name | Company | Catalog Number | Comments |
| Caliper ruler | Mauser | out of market | INOX Messchieber 0- 200 mm |
| Dissecting microscope or discarded ophthalmic surgery microscope | Möller Wedel | out of market | |
| Forceps | Fine Science | 11203-23 | SS INOX |
| HistoBond | Marienfeld, Germany | DIN ISO 8037-1 | coated slides |
| LED pipe for transillumination in a dark room | Schott, Mainz | out of market | KL150 |
| Microtome blade | Feather microtome blades | N/A | A35 type |
References
- Torczynski, E. Preparation of ocular specimens for histopathologic examination. Ophthalmology. 88 (12), 1367-1371 (1981).
- Atreya, A., Ateriya, N., Menezes, R. G. The eye in forensic practice: In the dead. Med Leg J. , (2024).
- Herwig-Carl, M. C., Holz, F. G., Löffler, K. U. Die Geschichte eines Auges – Untersuchung enukleierter Bulbi. Der Ophthalmologe. 117 (12), 1171-1179 (2020).
- Herwig-Carl, M. C., Holz, F., Löffler, K. U. Endophthalmitis aus ophthalmopathologischer Sicht. Klin Monbl Augenheilkd. 239 (7), 867-875 (2022).
- Herwig-Carl, M. C., Loeffler, K. U., Müller, A. M. Bedeutung der Untersuchung fetaler Augen : Ergänzung der Fetobduktion. Pathologe. 38 (4), 231-240 (2017).
- Herwig, M. C., Müller, A. M., Holz, F. G., Loeffler, K. U. Analyse eines ophthalmopathologischen Kollektivs humaner fetaler Augen unter besonderer Berücksichtigung außergewöhnlicher Befunde. Der Ophthalmologe. 107 (11), 1051-1058 (2010).
- Verdijk, R. M., Herwig-Carl, M. C. . Fetal and Neonatal Eye Pathology. , (2020).
- Breazzano, M. P., Unkrich, K. H., Barker-Griffith, A. E. Clinicopathological findings in abusive head trauma: analysis of 110 infant autopsy eyes. Am J Ophthalmol. 158 (6), 1146-1154.e2 (2014).
- Watts, P., et al. Abusive head trauma and the eye in infants and children - clinical guideline update by the royal college of ophthalmologists and the royal college of paediatrics and child health: executive summary. Eye (Lond). 38 (10), 1783-1786 (2024).
- Spencer, W. H. . Ophthalmic pathology: An atlas and textbook. , (1985).
- Khoshakhlagh, A. H., Mohammadzadeh, M., Sicard, P., Bamel, U. Human exposure to formaldehyde and health risk assessment: a 46-year systematic literature review. Environ Geochem Health. 46 (6), 206 (2024).
- Protano, C., et al. The carcinogenic effects of formaldehyde occupational exposure: A systematic review. Cancers. 14 (1), 165 (2021).
- Duong, A., Steinmaus, C., McHale, C. M., Vaughan, C. P., Zhang, L. Reproductive and developmental toxicity of formaldehyde: a systematic review. Mutat Res. 728 (3), 118-138 (2011).
- Ang, J. L., Collis, S., Dhillon, B., Cackett, P. The eye in forensic medicine: A narrative review. Asia Pac J Ophthalmol (Phila). 10 (5), 486-494 (2021).
- Gilliland, M. G. F., et al. Guidelines for postmortem protocol for ocular investigation of sudden unexplained infant death and suspected physical child abuse. Am J Forensic Med Pathol. 28 (4), 323-329 (2007).
- Bhardwaj, G., et al. A systematic review of the diagnostic accuracy of ocular signs in pediatric abusive head trauma. Ophthalmology. 117 (5), 983-992.e17 (2010).
- Herwig, M. C., Müller, A. M., Kuchelmeister, K., Loeffler, K. U. Pre- and intraretinal haemorrhages in a 22-week-old fetus of a mother suffering from HELLP syndrome and factor V Leiden mutation with deep vein thrombosis. Acta. 95 (1), e83-e84 (2017).
- Thau, A., et al. Retinal hemorrhage and bleeding disorders in children: A review. Child abuse Negl. 112, 104901 (2021).
- Mattheij, M., et al. Retinal haemorrhages in a university hospital: not always abusive head injury. Acta Neurol Belg. 117 (2), 515-522 (2017).
- Maguire, S. A., et al. Retinal haemorrhages and related findings in abusive and non-abusive head trauma: a systematic review. Eye (London). 27 (1), 28-36 (2013).
- Eagle, R. C. Optical coherence tomography: Clinicopathologic correlations - The 2016 Gordon K. Klintworth lecture. Ocul Oncol Pathol. 4 (4), 203-212 (2018).
- Stockinger, P., et al. Vergleichende In-vivo-/Ex-vivo-Bildgebung des hinteren Augenabschnitts - Version. Der Ophthalmologe. 118 (Suppl 2), 153-159 (2021).
- Agarwal, M., et al. Sympathetic ophthalmia - An Overview. Ocul Immunol Inflamm. 31 (4), 793-809 (2023).
- Albert, D., Syed, N. Protocol for the examination of specimens from patients with uveal melanoma: a basis for checklists. Arch Pathol Lab Med. 125 (9), 1177-1182 (2001).
- Albert, D., Syed, N. Protocol for the examination of specimens from patients with retinoblastoma: a basis for checklists. Arch Pathol Lab Med. 125 (9), 1183-1188 (2001).
- . American Academy of Ophthalmology (AAO) Available from: https://www.aao.org/education/image/gross-dissection-of-globe (2025)
- Lu, W., et al. Corrigendum to 'Recent progress in optical clearing of eye tissues' Exp. Eye Res (Nov; 2021) 212 108796. Exp Eye Res. 214, 108896 (2022).
- Lu, W., et al. Recent progress in optical clearing of eye tissues. Exp Eye Res. 212, 108796 (2021).
- Darche, M., et al. Three-dimensional characterization of developing and adult ocular vasculature in mice using in toto clearing. Commun Biol. 5 (1), 1135 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved