A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Corneal Confocal Microscopy: A Novel Non-invasive Technique to Quantify Small Fibre Pathology in Peripheral Neuropathies
* These authors contributed equally
In This Article
Summary
Corneal Confocal microscopy is a non-invasive clinical technique which may be used to quantify C fibre damage to diagnose and stratify patients with increasing neuropathic severity.
Abstract
The accurate quantification of peripheral neuropathy is important to define at risk patients, anticipate deterioration, and assess new therapies. Conventional methods assess neurological deficits and electrophysiology and quantitative sensory testing quantifies functional alterations to detect neuropathy. However, the earliest damage appears to be to the small fibres and yet these tests primarily assess large fibre dysfunction and have a limited ability to demonstrate regeneration and repair. The only techniques which allow a direct examination of unmyelinated nerve fibre damage and repair are sural nerve biopsy with electron microscopy and skin-punch biopsy. However, both are invasive procedures and require lengthy laboratory procedures and considerable expertise. Corneal Confocal microscopy is a non-invasive clinical technique which provides in-vivo imaging of corneal nerve fibres. We have demonstrated early nerve damage, which precedes loss of intraepidermal nerve fibres in skin biopsies together with stratification of neuropathic severity and repair following pancreas transplantation in diabetic patients. We have also demonstrated nerve damage in idiopathic small fibre neuropathy and Fabry's disease.
Protocol
Introduction:
The accurate quantification of peripheral neuropathy is important to define at risk patients, anticipate deterioration, and assess new therapies. Conventional methods assess neurological deficits and electrophysiology and quantitative sensory testing quantifies functional alterations to detect neuropathy. However, the earliest damage appears to be to the small fibres and yet these tests primarily assess large fibre dysfunction and have a limited ability to demonstrate regeneration and repair. The only techniques which allow a direct examination of unmyelinated nerve fibre damage and repair are sural nerve biopsy with electron microscopy and skin-punch biopsy. However, both are invasive procedures and require lengthy laboratory procedures and considerable expertise. Corneal Confocal microscopy is a non-invasive clinical technique which provides in-vivo imaging of corneal nerve fibres.
HRT III- Rostock Corneal Module (RCM)
Manual of Operation
1. Preparation of the Camera
The initial step in examination using HRT is preparation of the objective lens tip.
- First at the objective tube of the laser scanning camera, set the refraction to +12 diopters and then adjust the camera to the lowest position.
- Apply a large homogenous, bubble free, pea sized, drop of Viscotears on the lens tip.
- Remove a TomoCap from its sterile container and mount it over the lens tip such that the gel forms a meniscus between the objective lens and the cap. Push it as far as possible over the holder. Be careful not to touch the front surface of TomoCap during mounting.
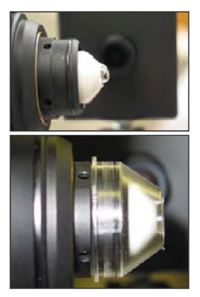
Figure 1. Preparation of the objective lens tip (top) applying viscotear gel (bottom) Applying the TomoCap. - Move the laser scanning camera as far backwards as possible on the camera mount.
- The inner and outer surface of the TomoCap both appear as a bright laser reflection. The focal plane adjustment wheel should be adjusted until a bright reflection is observed, indicating that the lens is focused within the front of the cap. The depth value shown in the focus position display should now be between -150 μm and +150 μm.
- Reset the depth setting to zero.
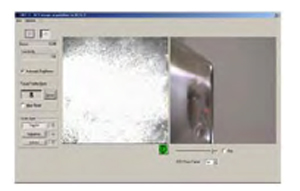
Figure 2. Adjustment of the focal plane.
2. Preparing the Patient
The subject's eyes are anaesthetised using a drop of 0.4% benoxinate hydrochloride, and Viscotears is used on the front of the eye for lubrication.
The subject is seated comfortably, with the chin on the chin rest and asked to press their forehead firmly against the forehead bar.

Figure 3. Preparing the patient
3. Aligning the Camera
It makes the examination easier if you instruct the patient to fix on the outer fixation light with the eye not being examined. The compact size of the microscope head means that the subject's contralateral eye is not obscured, enabling the use of distance targets.

Figure 4. Objective lens of the corneal module.
- Position the CCD camera such that its optical axis runs perpendicular to the optical axis of the laser scanning camera. The camera should be on the patient's right side when imaging the right eye and vice versa.
At this position, you should see the front surface of the TomoCap in the centre of the CCD camera live image. - Move the laser scanning camera towards the patient until the patient's cornea is at a distance of about 5 to 10 mm from the TomoCap, then move the laser scanning camera up/down and left/right using the black knobs on the camera mount until the TomoCap is positioned in the centre of the patient's cornea. At this stage you can check that the red laser light is in the centre of the cornea and for fine adjustment, observe the reflection of the laser beam from the cornea, which is visible in the CCD camera image. This reflection must occur exactly at the anterior pole of the cornea.

Figure 5. Alignment of the laser beam on the anterior pole of the cornea to capture central corneal images. - Ask the patient to open his/her eye as wide as possible. Move the laser scanning camera slowly forwards towards the patient until the TomoCap contacts the patient's cornea. If it is required to correct the position of the laser scanning camera, then move the camera away from the patient first, do the correction, then move it forward again to contact the cornea.

Figure 6. Demonstration of the thin gel bridge between the TomoCap and the cornea. - Minimal contact between the camera and the cornea is enough. In an optimal adjustment, a thin gel bridge between the TomoCap and the cornea is visible on the CCD camera live image.
If you press the TomoCap or move the cornea too close to the camera then you will see pressure on the cornea with a flattened appearance in the cornea via the CCD camera live image. Also in the images obtained a line showing the pressure will appear, therefore do not apply too much pressure to the cornea. Once contact has been established, do not move the position of the headrestto avoid sliding the cornea on the TomoCap.
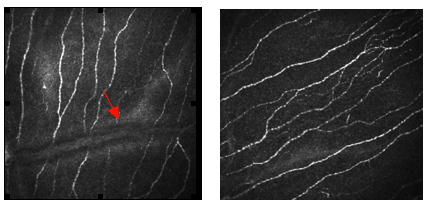
Figure 7. (left) Appearance of a straie due to application of excess pressure on the cornea by the TomoCap; (right) An image with normal appearance without excess pressure.
4. Examining the Patient
After the field of view has been selected, the HRT III laser camera is turned on and the image acquisition window appears on the screen. Use the CCD image to position the TomoCap and laser light on the patient's cornea. Examination using the selected mode may then be commenced.
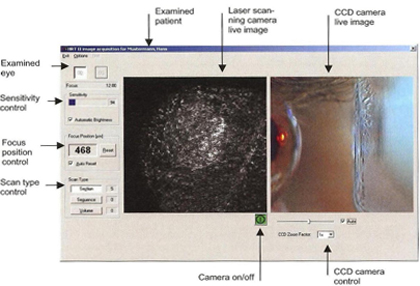
Figure 8. Viewing mode on monitor during acquisition of images.
The mode of acquisition
In the section mode, one single image is acquired and stored each time you press the foot switch. We use this mode for capturing images from the whole corneal layer including Bowman's layer in the centre of cornea. And then after capturing sufficient numbers of images, one can try the other modes including sequence and volume.

Figure 9. Display with choice for different image acquisition modes.
With the Sequence scan, one can acquire a sequence of up to 100 images with an adjustable frame rate. You can choose a frame rate between 30 frames and 1 frame per second (fps) and based on this mode it allows you to acquire a movie with a duration of 3 to 100 seconds.
In volume scan mode, the camera automatically acquires a series of 40 images at consecutive focal planes. A total depth of 85 μm can be scanned with the "FOV 400 μm" field lens and the focal distance between two consecutive images will be approximately 2.1 μm.
After a few scans the focus plane of lens can be changed to measure the depth of a corneal cell layer relative to the corneal surface by bringing the epithelial cell layer into focus and then clicking the Reset button to set the depth value to 0.
For the purpose of our present study, we obtain images from the central cornea at different depths. Therefore we do not move the scanning laser camera around the cornea horizontally or vertically, we just move backward and forward and capture images from all depths of Bowman's layer at the same corneal point. The distance between each image is approximately 1 μm; therefore if the depth of Bowman's is 10 μm, we will have 10 images. However for final analysis, we select images with a distance of approximately 2-3 μm between them.
After the examination is complete turn the camera off by clicking the camera power button in the acquisition window. Make the patient aware that he or she should not rub his or her eyes until the local anesthesia has worn off. As a precaution, you may wish to perform a slit lamp examination of the patient's cornea after the examination.
Remove the TomoCap from the camera and dispose it and then clean the microscope lens with a cotton swab moistened with distilled water.
5. Analyzing an Examination
a. Selecting the frames
To review and analyze existing examinations, the appropriate patient record(s) must be selected in the database window. The image viewing window provides an overview of existing cornea examinations for the selected patient. Examinations performed on different days are stored in separate visit tabs. The three different scan types are represented by three different icons in the HRT III cornea visit tab of the image viewing window of the Heidelberg Eye Explorer.
To survey the cornea image, double-click the appropriate image icon and the "Show" function opens the following window:
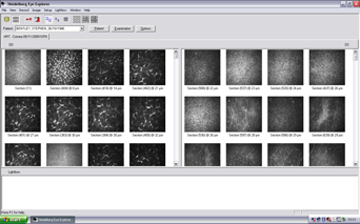
Figure 10. Display of all captured images after examination.

Figure 11. Display of selected image before export with CCD camera view of eye to show correct alignment.
To export the appropriate images for further analysis, click "Export" on the toolbar and then select the folder you want to save the images to.
We obtain and capture images from all layers of the cornea for each patient, but as the main focus of this research is on Bowman's layer and the corneal nerves, only the images from this layer will be analyzed. We select 5-6 frames from Bowman's layer for each subject at different depths of the cornea.

Figure 12. Display of all corneal layers from a healthy subject.
On average 5-6 frames from Bowman's layer in the centre of cornea at different depths are selected randomly for quantitative measurements and analysis.
b. Analyzing images
For analyzing the images captured from Bowman's layer of the central cornea with HRT III, we use specialized software that has been developed within our group called "CCM Image Analysis Tools v 0.6".
Four main parameters for corneal nerves are measured with this software:
- Nerve fibre density (NFD) which is defined as the number of main nerves/frame.
- Nerve branch density (NBD) number of main branches/frame.
- Nerve fibre length (NFL) which is the total length of all nerves/frame.
- Nerve fibre tortuosity (NFT) of the main nerves/frame.
To open CCM image, go to the file > open.
- Once the image is loaded, its size and type is displayed in the Image Information Box on the top left hand-side of the Control Panel.
- The default scale is displayed in the edit line of the image Information. If the scale is different, modify the number in the edit line.
- To start analyzing the image, select the parameter you want to measure from the Metric Box in the top right-hand side of the control panel.
- At the result box at the bottom of the page the number of NFD, NBD is presented in actual number, NFL in mm and tortuosity as a Tortuosity Coefficient (TC).
- However after exporting the results to an excel sheet, the results will be presented in no/mm2 for NFD and NBD and mm/mm2 for NFL.
- For this purpose after finishing measurements click on Add measurements, then go to File > Save as and then open the saved results with Excel.
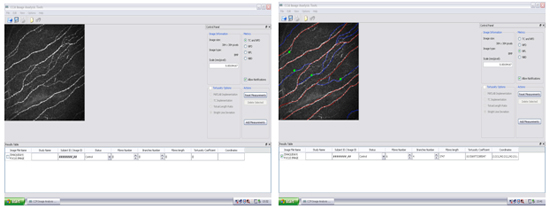
Figure 13. Display of corneal nerve image with tracing in analysis tool using "CCM Image Analysis Tool".
6. Representative Results:
We have demonstrated a progressive increasing in corneal nerve degeneration with increasing severity of diabetic neuropathy 1 and early nerve damage in diabetes which precedes loss of intraepidermal nerve fibres in skin biopsies 2 together with stratification of neuropathic severity and repair following pancreas transplantation 3 in diabetic patients. We have also demonstrated nerve damage in idiopathic small fibre neuropathy 4 and Fabry disease 5.

Figure 14. Corneal confocal microscopy images with HRT III of the corneal Bowman's layer (a) Control subject compared to (b) Patient with neuropathy and severe nerve damage.
Discussion
Corneal confocal microscopy (CCM) is a noninvasive clinical technique that may be used to detect early nerve damage and quantify small fibre pathology in peripheral neuropathies including diabetes, Fabry disease and idiopathic small fibre neuropathies (ISFN). The immediate clinical impact of this technique represents a huge leap forward in terms of our ability to diagnose, follow progression and assess therapeutic response in patients with diabetic neuropathy and other peripheral neuropathies and will have an immediate i...
Disclosures
Figures 4 - 6: Courtesy of Heidelberg Engineering
Acknowledgements
This work was supported by the Juvenile Diabetes Research Foundation International (grant 17-2008-1031) and the National Institutes of Health (grant 1R01DK077903-01A1).
Materials
| Name | Company | Catalog Number | Comments |
| HRT III CCM Machine - The Rostock Cornea Module | Heidelberg Engineering | ||
| CCM image analysis software v0.6 | University of Manchester |
References
- Abbott, C. A., Kallinikos, P., Marshall, A., Finnigan, J., Morgan, P., Efron, N., Boulton, A. J. M., Malik, R. A. Corneal Confocal Microscopy: A Novel Non-invasive Test to Diagnose and Assess Progression of Human Diabetic Neuropathy. Diabetes Care. 33, 1792-1797 (2010).
- Quattrini, C., Tavakoli, M., Jeziorska, M., Kallinikos, P., Tesfaye, S., Marshall, A., Finnigan, J., Boulton, A. J. M., Efron, N., Malik, R. A. Surrogate Markers of Small Fiber Damage in Human Diabetic Neuropathy. Diabetes. 56, 2148-2154 (2007).
- Mehra, S., Tavakoli, M., Efron, N., Boulton, A. J. M., Augustine, T., Malik, R. A. Corneal Confocal Microscopy Detects Early Nerve Regeneration After Pancreas Transplantation in Patients with Type 1 Diabetes. Diabetes Care. 30, 2608-2612 (2007).
- Tavakoli, M., Marshall, A., Pitceathly, R., Fadavi, H., Gow, D., Roberts, M. E., Efron, N., Boulton, A. J. M., Malik, R. A. Corneal confocal microscopy: A novel means to detect nerve fibre damage in patients with impaired glucose tolerance and idiopathic small fibre neuropathy. Experimental Neurology. 223, 245-250 (2010).
- Tavakoli, M., Marshall, A., Thompson, L., Kenny, M., Waldek, S., Efron, N., Malik, R. A. Corneal confocal microscopy: A novel technique to detect small-fibre neuropathy in patients with Fabry disease. Muscle & Nerve. 40, 976-984 (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved