A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Introduction to the Ultrasound Targeted Microbubble Destruction Technique
In This Article
Summary
Ultrasound Targeted Microbubble Destruction (UTMD) can be used to direct site-specific delivery of bioactive molecules, including therapeutic genes, to target organs accessible to ultrasound, such as the heart and liver1-6.
Abstract
In UTMD, bioactive molecules, such as negatively charged plasmid DNA vectors encoding a gene of interest, are added to the cationic shells of lipid microbubble contrast agents7-9. In mice these vector-carrying microbubbles can be administered intravenously or directly to the left ventricle of the heart. In larger animals they can also be infused through an intracoronary catheter. The subsequent delivery from the circulation to a target organ occurs by acoustic cavitation at a resonant frequency of the microbubbles. It seems likely that the mechanical energy generated by the microbubble destruction results in transient pore formation in or between the endothelial cells of the microvasculature of the targeted region10. As a result of this sonoporation effect, the transfection efficiency into and across the endothelial cells is enhanced, and transgene-encoding vectors are deposited into the surrounding tissue. Plasmid DNA remaining in the circulation is rapidly degraded by nucleases in the blood, which further reduces the likelihood of delivery to non-sonicated tissues and leads to highly specific target-organ transfection.
Protocol
1. Microbubble stock preparation
- In 10 mls of PBS mix 200 mg 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine and 50 mg 1,2-dipalmitoyl-sn-glycero-3-phosphatidylethanolamine with 1 g glucose.
- Heat the mixture in boiling water bath 20-30 minutes, pipette mixing every 5 minutes.
- The solution can be stored at 4°C for up to 6 months.
2. Microbubble Preparation
- Take a 250 μl of the prepared microbubble stock solution and incubate at 40°C for 15 minutes.
- The pre-warmed microbubble solution is then transferred to a 1.5 mL microtube containing 50 μl of glycerol.
- 1-2 mg of purified plasmid DNA encoding an expression construct for the gene of interest (purified in this example by Qiagen Endotoxin free MegaPrep kit, Qiagen, Germantown, MD, with a optimal concentration of 4mg/ml). 2.4) Phosphate-buffered saline is added to a final volume of 500 μl. Endotoxin free Qiagen maxipreps are used, as well as sterile PBS in order to ensure sterility.
- The air in the microtube is then replaced with Octafluoropropane gas.
- The microtube is then shaken vigorously in a dental amalgamator for 20 seconds.
- The subnatant containing residual DNA and buffer that has not bound to the microbubbles is then carefully removed and the microbubble layer is washed three times with sterile PBS to remove unattached DNA, and placed on ice between each wash cycle. We typically achieve a binding efficiency of 30-40%. All reagents are sterile and care is made to minimize contamination.
- The plasmid DNA-bound microbubbles are then placed on ice for up to two hours until use.
- The subnatant removed from the microtube after mixing and the PBS washes, can be used to determine the concentration of unbound DNA, and likewise the amount bound based upon the known initial concentration, by measuring the optical density of this solution at a wavelength of 260nm using a spectrophotometer.
3. Equipment Calibration
- Prior to first use, a 1 MHz cavitation transducer needs to be calibrated to ensure proper mechanical index and pulse repetition. A submergible 1 MHz, 13mm, unfocused transducer is connected to a 20 MHz Function/Arbitrary Waveform Generator through a power amplifier.
- The transducer is placed in a plastic container full of water, aimed directly at a hydrophone, which has been connected to a 500 MHz oscilloscope via a charge amplifier.
- Waveform, frequency, amplitude, burst cycle, and power amplification can all be modified to obtain the proper duty cycle and mechanical index optimal to cavitate the microbubbles. For this particular experiment, we have calibrated the system to a mechanical index equivalent to ~1.3 at 1 MHz.
4. Microbubble Delivery & UTMD
- Prior to microbubble delivery and UTMD, C57BL/6 mice were anesthetized with 100mg/kg ketamine and 5mg/kg xylazine through IP injection.
- Microbubble delivery was administered intravenously through a direct injection into the left ventricle of the heart or through tail vein catheterization. For the direct cardiac injection, a volume up to 100 μl of the plasmid DNA-loaded microbubble solution is injected through a 30 gauge needle inserted at the anterior 4th intercostal space under ultrasonic visualization into the left ventricle of the heart.
- The microbubble solution bolus resulting from the left interventricular injection is visualized using Visual Sonics’ 38 MHz high frequency ultrasound transducer placed in a stationary position on the thorax of the mouse in a long-axis view using VisualSonics’ Vevo 2100 Imaging System. All syringes and needles are sterile, insuring the most sterile environment possible for these injections.
- Immediately following the injection, microbubble destruction is carried out for ~5 minutes using a second, smaller sized low frequency 1.0 MHz transducer held directly over the desired organ, targeting destruction to this region. In this example, the ultrasound was administered to the liver at a pulse repetition frequency of 1.0 MHz, with a mechanical index equivalent to approximately 1.3-1.5, and pulse repetition period of 100 ms for every 20 cycles. Alternatively, the pulse can be gated to the ECG of the mouse (not shown in this experiment) to a burst of 3 frames of ultrasound, every 4-6 cardiac cycles. We obtain greater efficiency of transfection with protocols that allow the capillary bed to refill with bubbles between bursts of ultrasound.
5. Alternative Delivery Method
We chose to highlight the interventricular injection due to the complexity of the procedure, but in many instances, such as prolonged infusion of microbubbles, a tail vein injection is the preferred method. For the tail-vein method of microbubble delivery, the mouse is anesthetized the same way. A syringe containing the plasmid DNA-bound microbubbles is connected to a 27 gauge needle/tail vein catheter. The tail vein catheter is inserted into the distal third of either the right or left lateral veins that along the tail of the mouse. The syringe containing the microbubbles is placed in an infusion pump that automatically administers a uniform preset volume of solution over a preset period of time. We typically infuse 200-300μl at a rate of 3ml/hour.
Animal use
All animals were handled in accordance with good animal practice as defined by the relevant national and/or local animal welfare bodies, and all animal work was approved by the appropriate committee (University of Hawaii Institutional Animal Care and Use Committee, approval number 07-100-3). Appropriate anesthesia (ketamine/ zylazine) was used and analgesics (Bupivicaine and Buprenorphine) were available, though not required.
6. Representative Results:
The effectiveness of the UTMD-mediated plasmid DNA delivery can be evaluated through a variety of methods depending upon the genes encoded in the construct, such as, but not limited to; luciferase in vivo imaging, B-gal ex vivo staining, and/or immunohistology. In particular, in vivo bioluminescence imaging allows one to monitor the presence and duration of gene expression serially in mice transfected with a plasmid encoding a bioluminescent reporter gene (luciferase). The Xenogen In Vivo Imaging System (IVIS) (Caliper Life Sciences, Hopkinton, MA) is used for bioluminescent imaging. Images are typically taken of all mice the first day after UTMD mediated transfection and is repeated every three to four days until bioluminescent gene expression is no longer visually detectable through the system (Figure 1). To prepare mice for bioluminescent imaging, mice first receive an IP injection of the luciferase reporter probe D-luciferin (Caliper Life Sciences) and are then anesthetized ~3 minutes later. Biodistribution of the D-luciferin substrate is allowed to proceed for ~10 minutes before the animal is placed in the IVIS imaging chamber and a full body image scan is taken. During the acquisition, the photons emitted from the firefly luciferase/D-luciferin photochemical reaction are measured. Figure 1 also illustrates similar IVIS bioluminescent imaging of the liver following UTMD, and Figure 2 is an epifluorescence (100X) image of the transfected liver using an anti-luciferase primary antibody (Sigma-Aldrich) and AlexFluor-568 conjugated secondary antibody (Invitrogen). It is clear to see that the UTMD mediated liver transfection has affected not only the endothelial cells, but the hepatocyes as well.
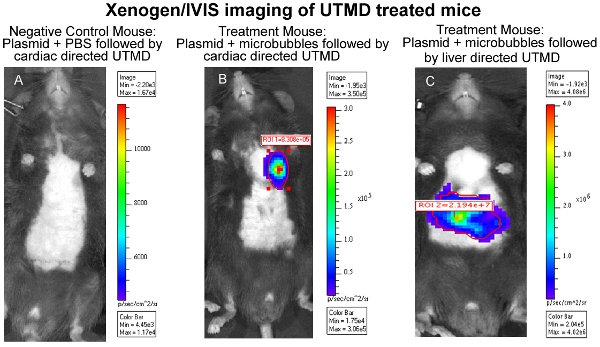
Figure 1. Xenogen/IVIS imaging of cardiac UTMD treated mice. (A) Negative Control Mouse: Plasmid + PBS
followed by cardiac directed UTMD, (B) Treatment Mouse: Plasmid + Microbubbles followed by cardiac directed UTMD, and (C) Treatment Mouse: Plasmid + Microbubbles followed by liver directed UTMD.
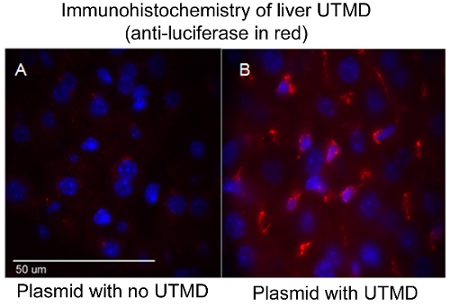
Figure 2. Immunohistochemistry of liver UTMD (anti-luciferase in red). (A) Plasmid with no UTMD,
and (B) Plasmid with UTMD. Confocal image (100X); nuclei are DAPI stained blue.
Discussion
UTMD represents a novel approach to gene delivery. As a platform technology it can be combined with any of the many potential gene therapy strategies, to deliver a myriad of bioactive molecules when a high degree of tissue specificity is desired. The main biological limitation of the technique is the low efficiency of transfection. Another important consideration is the accessibility of the target organ to ultrasound, which can be markedly diminished by intervening bone or air. The technique requires optimization of tech...
Disclosures
No conflicts of interest declared.
Acknowledgements
Grant support has included NHLBI HL080532, NHLBI HL073449, NCRR RR16453, and an AHA National Grant-in Aid Award (to RVS). A special thanks is extended to the Distance Course Design and Consulting (DCDC) group, dcdcgroup.org, for their assistance with video production and to the US Department of Education Grant No. P336C050047 that founded the DCDC.
Materials
| Name | Company | Catalog Number | Comments |
| 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine | Sigma-Aldrich | P-5911 | component of the microbubble lipid shell |
| 1,2-dipalmitoyl-sn-glycero-3-phosphatidylethanolamine | Sigma-Aldrich | P-3275 | component of the microbubble lipid shell |
| glucose | Sigma-Aldrich | G5400 | thought to stabilize the microbubbles |
| phosphate-buffered saline | Sigma-Aldrich | P5368 | |
| glycerol | Sigma-Aldrich | G5516 | believed to prevent microbubbles from coalescing |
| Octafluoropropane gas | Airgas | N/A | inert gas used in clinical applications |
| VialMix dental amalgamator | Bristol-Myers Squibb | N/A | |
| 1 MHz, 13mm, unfocused transducer | Olympus Corporation | A303S-SU | |
| 20 MHz Function/Arbitrary Waveform Generator | Agilent Technologies | 33220A | |
| Power Amplifier | Krohn-Hite Co. | Model 7500 | |
| Hydrophone | Bruel and Kjaer | Type 1803 | |
| Charge Amplifier | Bruel and Kjaer | Type 2634 | |
| 500 MHz Oscilloscope | LeCroy | 9354L | |
| VisualSonics’ Vevo 2100 Imaging System with 34 MHz transducer | VisualSonics, inc. | 2100 | |
| 27G one inch tail vein catheters | VisualSonics, inc. | N/A | |
| Genie Plus infusion pump | Kent Scientific | GENIE |
References
- Bekeredjian, R., Chen, S., Frenkel, P. A., Grayburn, P. A., Shohet, R. V. Ultrasound-targeted microbubble destruction can repeatedly direct highly specific plasmid expression to the heart. Circulation. 108, 1022-1026 (2003).
- Bekeredjian, R., Katus, H. A., Kuecherer, H. F. Therapeutic use of ultrasound targeted microbubble destruction: a review of non-cardiac applications. Ultraschall Med. 27, 134-140 (2006).
- Chen, S. Regeneration of pancreatic islets in vivo by ultrasound-targeted gene therapy. Gene Ther. 17, 1411-1420 (2010).
- Miao, C. H. Ultrasound enhances gene delivery of human factor IX plasmid. Hum Gene Ther. 16, 893-905 (2005).
- Shimoda, M., Chen, S., Noguchi, H., Matsumoto, S., Grayburn, P. A. In vivo non-viral gene delivery of human vascular endothelial growth factor improves revascularisation and restoration of euglycaemia after human islet transplantation into mouse liver. Diabetologia. 53, 1669-1679 (2010).
- Shohet, R. V. Echocardiographic destruction of albumin microbubbles directs gene delivery to the myocardium. Circulation. 101, 2554-2556 (2000).
- Sirsi, S., Borden, M. Microbubble Compositions, Properties and Biomedical Applications. Bubble Sci Eng Technol. 1, 3-17 (2009).
- Li, H. L. Ultrasound-targeted microbubble destruction enhances AAV-mediated gene transfection in human RPE cells in vitro and rat retina in vivo. Gene Ther. 16, 1146-1153 (2009).
- Lindner, J. R. Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov. 3, 527-532 (2004).
- Newman, C. M., Bettinger, T. Gene therapy progress and prospects: ultrasound for gene transfer. Gene Ther. 14, 465-475 (2007).
- Vancraeynest, D. Myocardial injury induced by ultrasound-targeted microbubble destruction: evidence for the contribution of myocardial ischemia. Ultrasound Med Biol. 35, 672-679 (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved