A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Synthesis of Nine-atom Deltahedral Zintl Ions of Germanium and their Functionalization with Organic Groups
In This Article
Summary
We present the high-temperature synthesis of intermetallic precursors K4Ge9, their dissolution in ethylenediamine to form Ge94- deltahedral Zintl ions, and the reaction of the clusters with alkynes to form organo-Zintl ions. The latter are characterized by electrospray mass spectrometry in solutions and by single-crystal X-ray diffraction in the solid state.
Abstract
Although the first studies of Zintl ions date between the late 1890's and early 1930's they were not structurally characterized until many years later.1,2 Their redox chemistry is even younger, just about ten years old, but despite this short history these deltahedral clusters ions E9n- (E = Si, Ge, Sn, Pb; n = 2, 3, 4) have already shown interesting and diverse reactivity and have been at the forefront of rapidly developing and exciting new chemistry.3-6 Notable milestones are the oxidative coupling of Ge94- clusters to oligomers and infinite chains,7-19 their metallation,14-16,20-25 capping by transition-metal organometallic fragments,26-34 insertion of a transition-metal atom at the center of the cluster which is sometimes combined with capping and oligomerization,35-47 addition of main-group organometallic fragments as exo-bonded substituents,48-50 and functionalization with various organic residues by reactions with organic halides and alkynes.51-58
This latter development of attaching organic fragments directly to the clusters has opened up a new field, namely organo-Zintl chemistry, that is potentially fertile for further synthetic explorations, and it is the step-by-step procedure for the synthesis of germanium-divinyl clusters described herein. The initial steps outline the synthesis of an intermetallic precursor of K4Ge9 from which the Ge94- clusters are extracted later in solution. This involves fused-silica glass blowing, arc-welding of niobium containers, and handling of highly air-sensitive materials in a glove box. The air-sensitive K4Ge9 is then dissolved in ethylenediamine in the box and then alkenylated by a reaction with Me3SiC≡CSiMe3. The reaction is followed by electrospray mass spectrometry while the resulting solution is used for obtaining single crystals containing the functionalized clusters [H2C=CH-Ge9-CH=CH2]2-. For this purpose the solution is centrifuged, filtered, and carefully layered with a toluene solution of 18-crown-6. Left undisturbed for a few days, the so-layered solutions produced orange crystalline blocks of [K(18-crown-6)]2[Ge9(HCCH2)2]•en which were characterized by single-crystal X-ray diffraction.
The process highlights standard reaction techniques, work-up, and analysis towards functionalized deltahedral Zintl clusters. It is hoped that it will help towards further development and understanding of these compounds in the community at large.
Protocol
1. Preparing Niobium Tubes
- Before cutting the niobium (Nb) tubes, prepare Nb-cleaning solution. In a 500 ml plastic bottle, measure out and add via a 100 ml measuring cylinder the following stock solutions as received: 110 ml H2SO4, followed by 50 ml HNO3, followed by 40 ml HF. Mix well and allow reaching room temperature before using.
- Measure the Nb tube, 4.5 cm in length, and cut with a pipe cutter. Avoid kinking the tubing. Repeat 3 more times.
- In a well-ventilated fume-hood, place the four Nb tubes, length-wise, in a 100 ml plastic beaker. Pour the Nb-cleaning solution into the beaker until tubes are completely covered. Close the sash of the fume-hood. Wait 12 to 20 seconds, or until brown nitrous oxide gas is released. Immediately fill the beaker with water and allow reaching room temperature.
- Use plastic thongs to remove Nb tubes, wash several times with water and then acetone, and dry them in a high temperature drying oven. (Dispose of the used acid solution after carefully neutralizing with KOH).
- Take the Nb tubes out of the oven and allow cooling down to room temperature. With vise-grips crimp about 1 cm of one end of each tube. Bend slightly the edge, about 1 mm.
- Turn on the vacuum pump and argon gas cylinder of the arc-welder. Clamp the four Nb tubes, in a staggered position, in the arc-welder's holder. Center the brass block (heat sink) in the arc-welder and slowly adjust niobium tubes on top. Close and clamp shut the door of arc welder.
- Adjust welding tip to height of niobium tubes. Turn on vacuum valve (ensure that argon refill valve is close). Allow vacuum to reach below 100 mm Hg (ideal: 30 mm Hg; wait time: 30 - 40 minutes) in order to completely evacuate chamber and Nb tubes of all air and moisture.
- When 30 - 60 mm Hg vacuum is achieved, close vacuum valve and slowly open the valve to argon cylinder to refill with argon to 7 in. Hg. Close argon gas valve. System should now be under partial vacuum.
- Put on certified rubber welding gloves. Position protective welding darkened glass over the window of the arc welder. Turn on the source set at 15 A (thicker niobium tubes use 20 - 55 A). As welding tip makes contact with niobium tube, welding arc forms, raise 1 cm over tube and slowly sweep above tube as niobium melts and seals completely. Repeat for remaining niobium tubes. Turn off power source and allow system to reach room temperature (15 - 30 min).
- Remove clamp from arc welder door. Slowly open argon gas valve and refill with argon until door is opened.
- Remove niobium tubes and label with electrical engraver: A, B, C, and D, respectively. Place back in drying oven until dry and place hot in antechamber of drybox, pumping under vacuum for 30 - 45 min.
- Refill small antechamber with high-purity nitrogen/argon gas. Bring four labeled niobium tubes into drybox. The containers are now ready to be loaded.
2. Loading Niobium Tubes: Preparing K4Ge9
- Tare the balance and then measure 156 mg K (4 mmol). With a spatula, carefully insert it into the Nb tube and push to the bottom.
- Weigh 653 mg of Ge (9 mmol) and insert into the tube on top of potassium metal.
- Carefully crimp the open end of the Nb tube using vise-grips and slightly bend the edge. Repeat for the three other Nb tubes. Place all four tubes in a jar [under inert gas], close the jar and place it in the box antechamber in order to bring out.
- Following steps 1.5 - 1.8, weld edges of four Nb tubes. The loaded tubes are now ready to be sealed in a fused silica tube.
3. Preparing Fused Silica Tube via Glass-Blowing
- Use circular glass saw to cut 10-14" pieces of large quartz tube (ID/OD = 20/22 mm) and medium quartz tube (7/9 mm). Cut also end of round ball joint. Wash thoroughly with water and acetone. Place in glass drying oven until completely dry. Take out of oven and allow reaching room temperature.
- Light the hydrogen/oxygen torch with a striker and slowly increase oxygen flow to get hot blue flame. Put on darkened safety glasses before inserting any quartz tubing into flame.
- Insert the large tube in the flame and slowly rotate one end allowing the tube to collapse to a bottle neck and then completely close. Using a spare medium quartz tube as a shaping rod, pull one end of the "white hot" (as seen through safety glasses) quartz tube to close the opening more quickly.
- After sealing opening, attach rubber septum with a blow hose at the open end and insert blow pipe into mouth (do not blow yet). While rotating the sealed end in the flame, slightly blow to keep positive pressure and prevent glass from collapsing. Next, a small hole is ready to be made in the center.
- Adjust gas flow to create a sharp blue flame and focus flame in center of closed end. When targeted spot becomes white hot, blow hard to create a large bubble or opening. Break bubble by gently scraping on lab bench. Insert opened hole into flame and open to 0.7 - 1cm while flaring edges with graphite reamer. This part (body) is now ready to be attached to the smaller tubing (neck).
- Close one end of the neck piece with rubber septum. Holding in one hand the body (with mouthpiece in mouth, be ready to blow) and in other hand the neck slowly rotate both ends simultaneously in the flame. Attach the ends and straighten outside of flame.
- Adjust size of flame to sharp one and slowly work out each quarter of sealed joint to ensure no holes and air bubbles. Keep positive pressure in order to prevent glass from collapsing. Remove the septum from top of neck. The neck is now ready to be attached to the ball joint.
- Using septum, close ball joint opening. Attach end of ball joint to neck following steps 3.5 - 3.6.
- Insert four Nb tubes into opened end of body. Attach end of blow hose to ball joint. Slowly close opened end of body by following step 3.3. Turn off flame and close all gas cylinders. Allow fused silica/quartz jacket to cool down.
- Pour dilute Nb cleaning solution (2 H20: 1 solution, refer to 1.1) into the silica tube and keep for 3 - 5 minutes until the Nb tubes are shiny and free of any oxidized areas. Rinse three times each with distilled water and acetone. Allow to dry completely. The niobium tubes are now in a fused silica/quartz jacket and ready to be evacuated and sealed off.
4. Sealing Fused Silica Tube using a High Vacuum Line
- Turn on the pump for the vacuum line. Fill dewars with liquid nitrogen. Turn on the mercury diffusion pump and the cooling water. Allow to reflux for a minimum of 30 minutes.
- Coat ball joint of fused silica jacket with high vacuum silicone grease. Attach to vacuum line. Evacuate tube completely for 30 min.
- Using a tesla coil, check for any leaks/holes.
- Put on Borosilicate Safety Glasses. Turn on small house gas/oxygen torch. Slowly pass flame from bottom of tube to neck, ensuring all air and moisture is gone.
- Slowly pass flame over Nb tubes, heating gently for 1 - 2 minutes. Careful not to get glass white hot as it will collapse as it is pulled in by high vacuum. Turn off torch and allow fused silica jacket to reach room temperature (15 - 30 min).
- Repeat step 4.4. Intensify flame to a sharp hot blue color. Slowly seal off tube below ball and neck joint. (Tip: can get entire glass area white hot and assist by slowing pulling off with other hand by holding end of silica jacket). Turn off torch.
- Turn off Hg-distillation. Allow to reach room temperature. Turn off water. Allow liquid nitrogen to slowly evaporate. The fused silica jacket is now ready to be placed in the furnace.
5. Heating the Reaction Mixtures in a Furnace
- Gently bring niobium tubes to center of quartz jacket and place directly over thermocouple. Insulate openings of furnace with glass wool. Turn on furnace and set at 950 °C for two days.
- After completion, using the glass-cutting circular saw (refer to 3.1) cut open the end of the fused silica tube and remove the Nb containers. Wash the niobium free of any debris with water and acetone and dry in the oven. Pump into the drybox.
- Using wire-cutting pliers cut the ends of the niobium tubes. Gently crush the coarse product. The fine intermetallic precursor is now ready to be brought into solution.
6. Dissolving Precursor in Ethylenediamine
- Weigh out 81 mg (0.1 mmol) of K4Ge9 into a test-tube. Add stir bar.
- Pipette in 2 - 2.5 ml of anhydrous ethylenediamine (previously distilled over sodium metal) and stir for 5 - 10 min at room temperature. A bright red solution forms.
- Note: If solution is stirred for too long, it will become green, indicative of oxidized clusters. A red solution is desired for functionalization.
7. Reacting Ge9-clusters with Me3SiC≡CSiMe3
- Slowly syringe in, drop-wise, 0.056 ml (0.25 mmol; a little over 2 equivalents) of Me3SiC≡CSiMe3. Oily layer is seen on top of the red cluster solution. As the reaction is stirred, the solution slowly becomes brown. Stir for four hours until a clear honey-brown solution is achieved.
- Place test-tube in centrifuge for 15 min. Carefully remove. Insert a glass fiber filter pipette (previously packed, dried at 180 °C overnight and pumped into drybox) into a clean test-tube.
- Carefully pipette the supernatant solution into the filter pipette and allow to filter. The clear honey-brown filtrate is now collected.
- Set aside 0.1 ml of filtrate in order to run an electrospray mass spectrometry (ES-MS) sample to confirm product before crystallization.
8. Running ES-MS of Reaction Solution
- Pump a clean 1 ml Hamilton syringe, syringe plunger and PEEK tubing into antechamber of drybox, for at least 30 - 45 min.
- Insert syringe plunger into syringe and fill with anhydrous ethylenediamine and dispense into a waste container. Purge the syringe two more times.
- Fill the syringe again with anhydrous ethylenediamine and push through the PEEK tubing. Purge the PEEK tubing two more times. The PEEK tubing is now filled with anhydrous ethylenediamine.
- Fill the empty syringe with the filtered Ge9-divinyl solution. (Tip: this can be placed in a nitrogen filled Ziploc bag for transportation if mass spectrometer is outside of the drybox). Bring out of drybox.
- Place syringe attached to PEEK tubing in Harvard syringe pump at 10 μL/min. Attach PEEK tubing securely to electrospray mass spectrometer. Collect spectrum in the negative ion mode on a Micromass Quattro-LC triple quadrupole mass spectrometer (typical conditions: 100 °C source temperature, 125 °C desolvation temperature, 2.5 kV capillary voltage, 30 - 65 V cone voltage) or on a Bruker Microtof-II mass spectrometer (capillary at 3800 V, nebulizer at 0.6 Bar, desolvation temperature at 190 °C, capillary exit at 100 V, detector at 1200 V). (Note: samples are highly air and moisture sensitive, purging the chamber for 15 - 60 min before running samples is preferred.)
- Resulting spectrum will show isotope pattern for the functionalized clusters (see Figure 1). The remaining filtrate in the drybox can now be crystallized.
9. Crystallizing Ge9-divinyl Ions with a Sequestering Agent
- Pipette remaining filtrate into two equal aliquots in two clean test-tubes. Label test-tubes C and D (as an example). Set aside.
- Weigh 0.4 mmol (105 mg) of anhydrous 18-crown-6 into a clean test-tube and add 8 ml of toluene. Mix well until completely dissolved.
- Layering with Toluene, Method A: Gently pipette 4 ml of this solution on top of the test-tube C and D. (Tip: Try to achieve two separate phases.) Place a rubber stopper on each test-tube. Set aside undisturbed in a test-tube rack to crystallize.
- Layering with Toluene, Method B: Pipette 4ml of 18-crown-6/toluene solution into two clean test-tube labeled E and D. Insert a filter pipette (note: if solution needs to be filtered again) or a regular pipette into test-tube E and F. Pipette the solution of test-tube C into E and test-tube D into F. (Note: this reverse layering will quickly result in two phases.) Place a rubber stopper on each test-tube. Set aside undisturbed in a test-tube rack to crystallize.
- Bright orange blocks should crystallize within 1 - 3 days. The unit cell of the crystals can then be confirmed by single crystal X-ray diffraction.
10. Checking Crystals Unit Cell on a D8-Diffractometer
- Fill a plastic bottle with Paratone-N oil and allow all air bubbles to be removed. Completely degas the oil in antechamber of drybox, under vacuum, overnight. Bring into the drybox.
- Apply 2 - 3 drops to glass slide. Coat spatula tip in the oil and insert into crystallizing test-tube. Select orange crystals from the test-tube and immersed in the Paratone-N oil. (Note: ensure all air-sensitive crystals are coated in oil). Bring outside drybox.
- Using a high resolution microscope, select a single crystal and drag to the edge of the oil on the slide with a straight stainless probe.
- Carefully remove excess oil from the crystal by dragging to edge of glass slide. Mount single crystal on Mitegen micro mount loop and quickly position under cold stream (100 K) of the Bruker D8 APEX-II diffractometer equipped with CCD area detector using graphite-monochromated Mo Kα radiation.
- Ensure good high angle diffractions and acquire unit cell.
- Compare and confirm unit cell to that of [K(18-crown-6)]2[Ge9(HCCH2)2]•en, 1, triclinic, P-1, a = 10.974(4), b = 14.3863(5), and c = 16.2272(6) Å, α = 85.946(2), β = 71.136(2), and γ = 89.264(2) °, V = 2412.21(15) Å3, Z =2. 53
11. Representative Results
The unique isotope pattern of the anionic clusters allows them to be easily detected in the negative ion-mode (Fig 1). Also noteworthy is that reduced singly charged species, in addition to pairing with a potassium ion is a common phenomenon of this soft-ionization technique.59
The crystal structure with relevant bond lengths and angles of [Ge9(CH=CH2)2]2- in [K(18-crown-6)]2[Ge9(HCCH2)2]•en, 1, can be seen in Figure 2 .
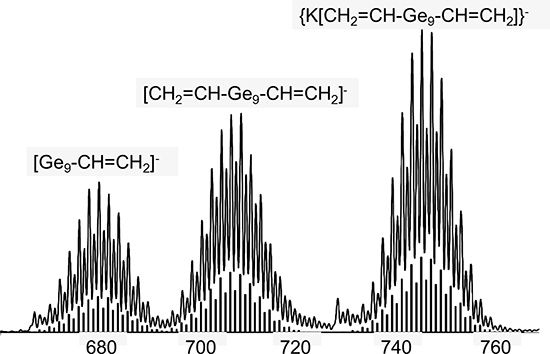
Figure 1. ES-MS spectra (negative-ion mode) of ethylenediamine solutions of the reactions of Ge9 clusters with Me3SiC≡CSiMe3. Shown are also the theoretical isotope distributions below the experimental distribution. (Sevov et. al. Inorg. Chem. 2007, 46, 10953.)
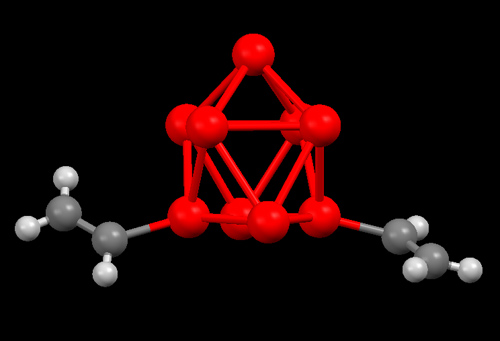
Figure 2. A view of [K(18-crown-6)]2[Ge9(HCCH2)2]•en, 1. Color scheme:  = Ge,
= Ge,  = C,
= C,  = H. Selected bond lengths and angles: Ge-C 1.961 and 1.950 Å, C=C 1.318 and 1.316 Å, Ge-C-C 123 and 127 °. (Sevov et. al. Inorg. Chem. 2007, 46, 10953.)
= H. Selected bond lengths and angles: Ge-C 1.961 and 1.950 Å, C=C 1.318 and 1.316 Å, Ge-C-C 123 and 127 °. (Sevov et. al. Inorg. Chem. 2007, 46, 10953.)

Figure 3. Schematic representation of Preparing Niobium Tubes: (a) cutting Nb tubes; (b) cleaning Nb tubes in a Nb acid solution; (c) using vise-grips to crimp and bend Nb tube.
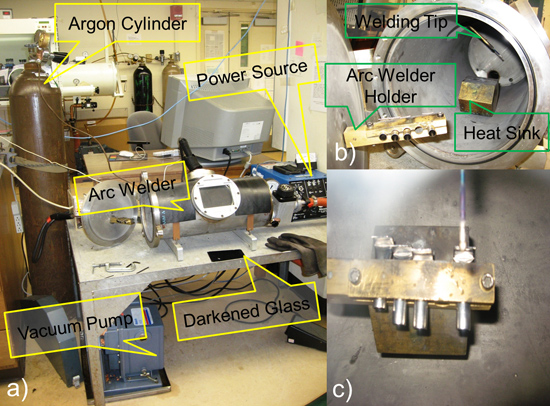
Figure 4. Schematic representation of Preparing Niobium Tubes: (a) diagram of arc welder; (b) staggered Nb tubes in arc welder holder and (c) welding tip above Nb tubes.
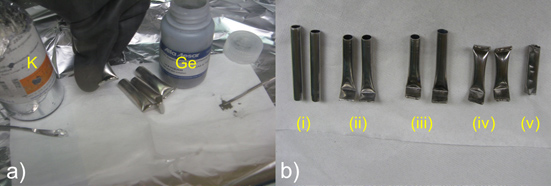
Figure 5. Schematic representation of Loading Niobium Tubes : (a) inside the drybox and (b) Nb tubes: (i) before welding, (ii) after using vise-grips to crimp one edge, (iii) after welding one edge, (iv) after loading and then welding the Nb tube closed, (v) after opening the Nb tube to take out the K4Ge9 precursor.
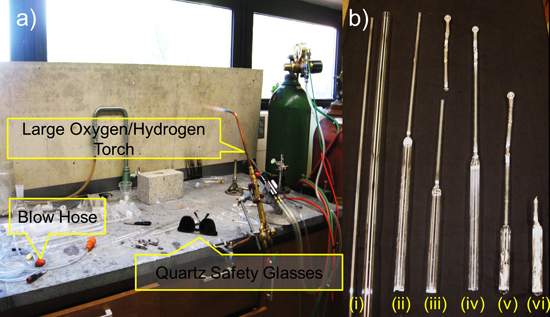
Figure 6. Schematic representation of Preparing Fused-Silica Tube by Glass-Blowing in (a) and (b)(i) large and small quartz tubing, (ii) body and neck sealed together, (iii) neck with ball joint, (iv) neck and ball joint sealed together, (v) Nb tubes sealed inside quartz tube, (vi) after the fused silica tube is sealed.
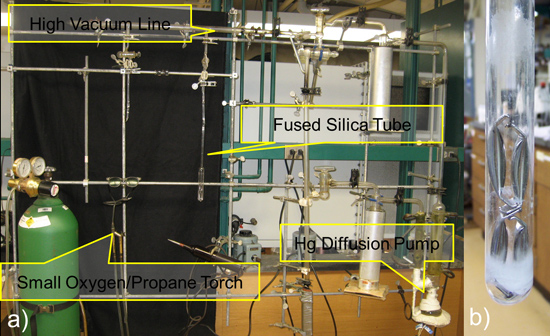
Figure 7. Schematic representation of Sealing Fused Silica Tubes on a High Vacuum Line in (a) and (b) after the Nb tubes is sealed showing etching of the quartz tube from Nb acid solution.

Figure 8. Schematic representation of Placing Loaded Fused Silica Tubes in Furnace.
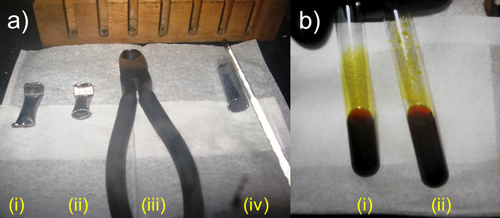
Figure 9. Schematic representation of Reacting K4Ge9 with Me3SiC≡CSiMe3 inside the drybox (a) (i) unopened Nb tube, (ii) one edge of the Nb tube cut with the (iii) cutting pliers, (iv) crushed precursor and (b) (i) precursor dissolved in ethylenediamine, (ii) immediately after Me3SiC≡CSiMe3 is added (oily droplets on top of test-tube walls seen).

Figure 10. Schematic representation of Running ES-MS of Reaction Solution in (a) mass spectrometer syringe prepared in dry box, (b) Bruker Microtof-II.
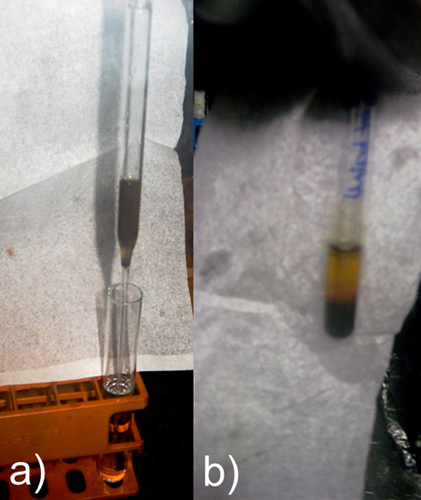
Figure 11. Schematic representation of Crystallizing Ge9-divinyl with Sequestering Agents in (a) reverse layering and (b) several hours later.
Figure 12. Schematic representation of Checking Crystals Unit Cell on a D8-Diffractometer: (a) selecting crystals under the microscope and (b) collecting a unit cell.
Access restricted. Please log in or start a trial to view this content.
Discussion
It is important to clean well the partially oxidized Nb tubes. However, if the tubes are left too long in the Nb cleaning solution, this will severely compromise the thickness of the tube. Thus, 10 – 15 seconds are imperative and the tubes should be very lustrous at the end (Fig 3). After the tubes are sealed inside the fused silica jacket they should be cleaned again with a dilute Nb acid solution. This should result in mild effervescence, cleaning any oxidized areas on Nb tubes that occurred during welding o...
Access restricted. Please log in or start a trial to view this content.
Disclosures
No conflicts of interest declared.
Acknowledgements
The authors would like to thank the National Science Foundation for the continuous financial support (CHE-0742365) and for the purchase of a Bruker APEX II diffractometer (CHE-0443233) and a Bruker Microtof-II mass spectrometer (CHE-0741793). The authors would also like to thank CEST facility for their use of the Micromass Quattro-LC mass spectrometer.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| D8-Xray diffractometer | Bruker Corporation | Bruker APEX II | |
| Electrospray mass spectrometer | Bruker Corporation | Microtof-II | |
| Electrospray mass spectrometer | Micromass | Quattro-LC | triple -quadropole |
| Drybox | Innovative Technology | S-1-M-DL | IT-Sys1 model |
| Inert Gas/Vacuum Shielded Arc Welding Arrangement |  LDS Vacuum Products LDS Vacuum Products | Special Order | |
| Arc Welder Power Source | Miller | Maxstar-91 | |
| Welding Rubber Gloves | Home Depot | KH643 | |
| Electric Engraver | Burgess Products | 74 | Vibro-Graver |
| Circular Glass Saw | Pistorius Machine Co. Inc | GC-12-B | |
| Tube Furnace | Lindberg/Blue M | TF55035 | Minimite Laboratory Tube Furnace, Moldatherm (1100 °C) |
| Glass Drying Oven | Fisher Scientific | 13-247-650G | |
| High Vacuum Hg Schlenk-Line | Special Order | Univ Of Notre Dame | Alternative: Edwards E050/60; VWR International; Cat. No. EVB302-07-110 |
| Large Torch | Victor Technologies | JT100C | Welding torch, tip: Victor 5-W-J |
| Small Torch | Veriflo Co. | 3A | Blow-pipe |
| Tesla Coil | VWR international | KT691550-0000 | Leak detector |
| Stirrer/Hot -Plate | VWR international | 12620-970 | VWR HOT PLATE STR DY-DUAL120V |
| Balance | Denver Instrument | 100A | XE Series |
| Centrifuge | LW Scientific, Inc. | E8C-08AV-1501 | Variable speed |
| Graphite Reamer, (flaring) | ABR Imagery, Inc. | 850-523 B01 | Open holes in Glass Blowing and flaring edges |
| Striker | Fisher Scientific | 12-007 | |
| Vise-Grips | Home Depot | 0902L3SM | |
| Pipe-Cutter | Home Depot | 32820 | |
| Cutting Pliers | Home Depot | 437 | |
| Plastic Beaker | VWR international | 13890-046 | |
| Measuring Cylinder | VWR international | 65000-006 | Careful, HF etches glass (if using a glass one) |
| Large Plastic Bottle | VWR international | 16128-542 | |
| 13 x 100 Test-Tubes | VWR international | 47729-572 | CULTURE TUBE 13X100 CS1000 |
| Laboratory (Rubber) Stoppers | Sigma-Aldrich | Z164437-100EA | Size 00 |
| Test-Tube Rack | VWR international | 60196-702 | 10-13 mm tube OD |
| Stir-Bars | StirBars.com/Big Science Inc. | SBM-0803-MIC | PTFE 8x3 mm Micro |
| Glass Pipettes | VWR international | 14673-043 | VWR PIPET PASTEUR 9IN CS1000 |
| Rubber Bulbs | VWR international | 56311-062 | Latex, thin walled |
| Glass Wool | Unifrax I LLC | 6048 | Fiberfrax Bulk Fiber Insulation, Ceramic fiber |
| Glass Slides | VWR international | 16004-422 | 75x25x1mm, Microscope Slides |
| Paratone-N oil | Hampton Research | Parabar 10312 | Known as: Paratone-N, Paratone-8277, Infineum V8512 |
| High Vacuum Silicone Grease | VWR international | 59344-055 | Dow Corning |
| Liquid Nitrogen | University of Notre Dame | ||
| Argon Gas Cylinder | Praxair, Inc. | TARGHP | |
| Nitrogen Gas Cylinder | Praxair, Inc. | QNITPP | |
| Oxygen Gas Cylinder | Praxair, Inc. | OT | 337 cf CYL |
| Hydrogen Gas Cylinder | Praxair, Inc. | HK | 195 cf CYL |
| Propane Gas Cylinder/source | University of Notre Dame | UND | |
| Quartz tubing, Lg | Quartz Scientific Inc. | 100020B | 20 mm id x 22mm od x 48" clear fused quart tubing |
| Quartz tubing, Md | Quartz Scientific Inc. | 100007B | Clear Fused Quartz Tubing,7mm id x 9mm od x 48" |
| Round Bottom Quartz Joint | Quartz Scientific Inc. | 6160189B | Ball joint |
| Quartz Safety Glasses | Wale Apparatus | 11-1127 | waleapparatus.com |
| Pyrex Safety Glasses | Wale Apparatus | 11-2125-B3 | For clear and color borosilicate glass |
| Blow Hose Kit | Glass House | BH020 | glasshousesupply.com |
| Niobium Tubes | Shaanxi Tony Metals Co., Ltd | Niobium Tube, 50 ft | Seamless Niobium Tube Outside diameter: 0.375 (±0.005) inches. Wall thickness: 0.02(±0.003) Inches Niobium should be annealed. |
| PEEK Starter Kit for Mass Spect | Waters | PSL613321 | PEEK (PolyEtherEtherKetone) tubing, nuts, ferrule, fits |
| Mass Spect Needle Set | VWR international | 60373-992 | Hamilton Manufacturer (81165) |
| H2SO4 | VWR international | BDH3072-2.5LG | ACS Grade |
| HNO3 | VWR international | BDH3046-2.5LPC | ACS Grade |
| HF | VWR international | BDH3040-500MLP | ACS Grade |
| Distilled Water | University of Notre Dame | UND | |
| Acetone | VWR international | BDH1101-4LP | |
| Ethylenediamine | VWR international | AAA12132-0F | 99% 2.5 L |
| Toluene | VWR international | 200004-418 | 99.8 %, anhydrous |
| Mercury | Strem Chemicals, Inc. | 93-8046 | |
| Potassium (K) metal | Strem Chemicals, Inc. | 19-1989 | Sealed in glass ampoule under Ar |
| Germanium (Ge) powder | VWR international | AA10190-18 | GERM PWR -100 MESH 99.999% 50G |
| Bistrimetylsilylacetylene, (Me3SiC≡CCSiMe3) | Fisher Scientific | AC182010100 | |
| 18-crown-6 (1,4,7,10,13,16-Hexaoxacyclooctadecane) | VWR international | 200001-954 | 99%, 25 gm |
| 2,2,2-crypt (4,7,13,16,21,24-Hexaoxa-1,10 diazabicyclo[8.8.8]hexacosane) | Sigma-Aldrich | 291110-1G | 98% |
References
- Corbett, J. D. Polyatomic Zintl Anions of the Post-Transition Elements. Chem. Rev. 85, 383-397 (1985).
- Fässler, T. F. The renaissance of homoatomic nine-atom polyhedral of the heavier carbon-group elements Si-Pb. Coord. Chem. Rev. 215, 347-377 (2001).
- Sevov, S. C., Goicoechea, J. M. Chemistry of Deltahedral Zintl Ions. Organometallics. 25, 5678-5692 (2006).
- Sevov, S. C. Tin Chemistry: Fundamentals, Frontiers and Applications. Davies, A. G. , John Wiley & Sons. Chichester, UK. 138-151 (2008).
- Scharfe, S., Fässler, T. F. Polyhedral nine-atom clusters of tetrel elements and intermetalloid derivatives. Phil. Trans. R. Soc. A. , 368-1265 (2010).
- Scharfe, S., Kraus, F., Stegmaier, S., Schier, A., Fässler, T. F. Zintl Ions, Cage Compounds, and Intermetalloid Clusters of Group 14 and Group 15 Elements. Angew. Chem. Int. Ed. 50, 3630-3670 (2011).
- Xu, L., Sevov, S. C. Oxidative Coupling of Deltahedral [Ge9]4- Zintl Ions. J. Am. Chem. Soc. 121, 9245-9246 (1999).
- Hauptmann, R., Fässler, T. F. Low Dimensional Arrangements of the Zintl Ion [Ge9-Ge9]6- and Chemical Bonding in [Ge6]2-, [Ge9=Ge9]6- and 1∞{[Ge9]}2-. Z. Anorg. Allg. Chem. 629, 2266-2273 (2003).
- Suchentrunk, C., Daniels, J., Somer, M., Carrillo-Cabrera, W., Korber, N. Synthesis and Crystal Structures of the Polygermanide Ammoniates K4Ge9•9NH3, Rb4Ge9•5NH3 and Cs6Ge18•4NH3. Z. Naturforsch. 60b, 277-283 (2005).
- Ugrinov, A., Sevov, S. C. Ge9=Ge9=Ge9]6-: A Linear Trimer of 27 Germanium Atoms. J. Am. Chem. Soc. 124, 10990-10991 (2002).
- Yong, L., Hoffmann, S. D., Fässler, T. F. The Controlled Oxidative coupling of Ge94- Zintl Anions to a Linear Trimer [Ge9=Ge9=Ge9]6. Z. Anorg. Allg. Chem. 631, 1149-1153 (2005).
- Ugrinov, A., Sevov, S. C. Ge9=Ge9=Ge9=Ge9]8-: A Linear Tetramer of Nine-Atom Germanium Clusters, a Nanorod. Inorg. Chem. 42, 5789-5791 (2003).
- Yong, L., Hoffmann, S. D., Fässler, T. F. Oxidative Coupling of Ge94- Zintl Anions – Hexagonal Rod Packing of Linear [Ge9=Ge9=Ge9=Ge9]8-. Z. Anorg. Allg. Chem. 630, 1977-1981 (2004).
- Denning, M. S., Goicoechea, J. M. [Hg3(Ge9)4]10-: a nanometric molecular rod precursor to polymeric mercury-linked cluster chains. Dalton Trans. , 5882-5885 (2008).
- Boeddinghaus, M. B., Hoffmann, S. D., Fässler, T. F. Synthesis and Crystal Structure of [K([2,2,2]crypt)]2[HgGe9](dmf). Z. Annorg. Allg. Chem. 633, 2338-2341 (2007).
- Nienhaus, A., Hauptmann, R., Fässler, T. F. 1∞[HgGe9]2- --A Polymer with Zintl Ions as Building Blocks Covalently Linked by Heteroatoms. Angew. Chem., Int. Ed. 41, 3213-3215 (2002).
- Downie, C., Tang, Z., Guloy, A. M. An Unprecedented 1∞[Ge9]2- Polymer: A Link between Molecular Zintl Clusters and Solid-State Phases. Angew. Chem., Int. Ed. 39, 337-340 (2000).
- Downie, C., Mao, J. -G., Parmar, H., Guloy, A. M. The Role of Sequestering Agents in the Formation and Structure of Germanium Anion Cluster Polymers. Inorg. Chem. 43, 1992-1997 (2004).
- Ugrinov, A., Sevov, S. C. Synthesis of a chain of nine-atom germanium clusters accompanied with dimerization of the sequestering. 8, 1878-1882 (2005).
- Spiekermann, A., Hoffmann, S. D., Kraus, F., Fässler, T. F. Au3Ge18]5- – Gold-Germanium Cluster with Remarkable Au-Au Interactions. Angew. Chem., Int. Ed. 46, 1638-1640 (2007).
- Spiekermann, A., Hoffmann, S. D., Fässler, T. F., Krossing, I., Preiss, U. [Au3Ge45]9-–A Binary Anion Containing a {Ge45}. Cluster. Angew. Chem., Int. Ed. 46, 5310-5313 (2007).
- Wang, J. -Q., Wahl, B., Fässler, T. F. [Ag(Sn9-Sn9)]5-: A Homoleptic Silver Complex of A Dimeric Sn9 Zintl Anion. Angew. Chem., Int. Ed. 49, 6592-6595 (2010).
- Scharfe, S., Fässler, T. F. VVarying Bonding Modes of the Zintl Ion [Ge9]4- in CuI Complexes: Syntheses and Structures of [Cu(η4-Ge9)(PR3)]3- (R = iPr, Cy) and [Cu(η4-Ge9)(η1-Ge9)]7-. Eur. J. Inorg. Chem. 8, 1207-1213 (2010).
- Yong, L., Boeddinghaus, M. B., Fässler, T. F. [Sn9HgSn9]6-: An Intermetalloid Zintl Ion with Two Sn9 Connected by Heteroatom. Z. Anorg. Allg. Chem. 636, 1293-1296 (2010).
- Rios, D., Gillett-Kunnath, M. M., Taylor, J. D., Oliver, A. G., Sevov, S. C. Addition of a Thallium Vertex to Empty and Centered Nine-Atom Deltahedral Zintl Ions of Germanium and Tin. Inorg. Chem. 50, 2373-2377 (2011).
- Eichhorn, B. W., Haushalter, R. C. Synthesis and Structure of closo-Sn9Cr(CO)34-: The First Member in a New Class of Polyhedral Clusters. J. Amer. Chem. Soc. 110, 8704-8706 (1988).
- Eichhorn, B. W., Haushalter, R. C. closo-[CrPb9(CO)3]4-: a 100 Year History of the Nonaplumbide Tetra-anion. J. Chem. Soc. Chem. Commun. , 937-938 (1990).
- Kesanli, B., Fettinger, J., Eichhorn, B. W. The closo-[Sn9M(CO)3]4- Zintl Ion Clusters where M = Cr, Mo, W: Two Structural Isomers and Their Dynamic Behavior. Chem. Eur. J. 7, 5277-5285 (2001).
- Kesanli, B., Fettinger, J., Gardner, D. R., Eichhorn, B. The [Sn9Pt2(PPh3)]2- and [Sn9Ni2(CO)]3- Complexes: Two Markedly Different Sn9M2L Transition Metal Zintl Ion Clusters and Their Dynamic. 124, 4779-4788 (2002).
- Campbell, J., Mercier, H. P. A., Holger, F., Santry, D. P., Dixon, D. A., Schrobilgen, G. J. Syntheses, Crystal Structures, and Density Functional Theory Calculations of the closo-[1-M(CO)3(η4-E9)4- (E = Sn, Pb; M = Mo, W) Cluster Anions and Solution NMR Spectroscopic Characterization of [1-M(CO)3(η4-Sn9)4- (M = Cr, Mo, W). Inorg. Chem. 41, 86-107 (2002).
- Yong, L., Hoffmann, S. D., Fässler, T. F. Crystal Structures of [K(2.2.2-crypt)]4[Pb9Mo(CO)3]–Isolation of the Novel Isomers [(η5-Pb9)Mo(CO)3]4- beside [(η4-Pb9)Mo(CO)3]4. Eur. J. Inorg. Chem. , 3663-3669 (2005).
- Esenturk, E. N., Fettinger, J., Eichhorn, B. Synthesis and characterization of the [Ni6Ge13(CO)5]4- and [Ge9Ni2(PPh3)]2- Zintl ion clusters. Polyhedron. 25, 521-529 (2006).
- Rios, D., Sevov, S. C. The Elusive closo-Ge102- Zintl Ion: Finally "Captured" as a Ligand in the Complex [Ge10Mn(CO)4]3-. Inorg. Chem. 49, 6396-6398 (2010).
- Downing, D. O., Zavalij, P., Eichhorn, B. W. The closo-[Sn9Ir(cod)]3- and [Pb9Ir(cod)]3- Zintl Ions: Isostructural IrI Derivatives of the nido-E94- Anions (E = Sn, Pb). Eur. J. Inorg. Chem. , 890-894 (2010).
- Esenturk, E. N., Fettinger, J., Lam, Y. -F., Eichhorn, B. Pt@Pb12]2-. Angew. Chem. Int. Ed. 43, 2132-2134 (2004).
- Goicoechea, J. M., Sevov, S. C. [(Ni-Ni-Ni)@(Ge9)2]4-: A Linear triatomic Nickel Filament Enclosed in a Dimer of Nine-Atom Germanium Clusters. Angew. Chem. Int. Ed. 44, 4026-4028 (2005).
- Goicoechea, J. M., Sevov, S. C. [(Pd-Pd)@Ge18]4-: A Palladium Dimer Inside the Largest Single-Cage Deltahedron. J. Am. Chem. Soc. 127, 7676-7677 (2005).
- Esenturk, E. N., Fettinger, J., Eichhorn, B. The closo-Pb102- Zintl ion in the [Ni@Pb10]2 cluster. Chem. Commun. , 247-249 (2005).
- Goicoechea, J. M., Sevov, S. C. Deltahedral Germanium Clusters: Insertion of Transition-Metal Atoms and Addition of Organometallic Fragments. J. Am. Chem. Soc. 128, 4155-4161 (2006).
- Esenturk, E. N., Fettinger, J., Eichhorn, B. W. Synthesis, Structure, and Dynamic Properties of [Ni2Sn17]4. J. Am. Chem. Soc. 128, 12-13 (2006).
- Esenturk, E. N., Fettinger, J., Eichhorn, B. W. The Pb122- and Pb102- Zintl Ions and the M@Pb122- and M@Pb102- Cluster Series Where M = Ni, Pd, Pt. J. Am. Chem. Soc. 128, 9178-9186 (2006).
- Kocak, F. S., Zavalij, P., Lam, Y. F., Eichhorn, B. W. Solution Dynamics and Gas-Phase Chemistry of Pd2@Sn184. Inorg. Chem. 47, 3515-3520 (2008).
- Scharfe, S., Fässler, T. F., Stegmaier, S., Hoffmann, S. D., Ruhland, K. [Cu@Sn9]3- and [Cu@Pb9]3-: Intermetalloid Clusters with Endohedral Cu Atoms in Spherical Environments. Chem. Eur. J. 14, 4479-4483 (2008).
- Zhou, B., Denning, M. S., Kays, D. L., Goicoechea, J. M. Synthesis and Isolation of [Fe@Ge10]3-: A Pentagonal Prismatic Zintl Ion Cage Encapsulating an Interstitial Iron Atom. J. Am. Chem. Soc. 131, 2802-2803 (2009).
- Wang, J. -Q., Stegmaier, S., Fässler, T. F. [Co@Ge10]3-: An Intermetalloid Cluster with Archimedean Pentagonal Prismatic Structure. Angew. Chem. Int. Ed. 48, 1998-2002 (2009).
- Wang, J. -Q., Stegmaier, S., Wahl, B., Fässler, T. F. Step-by-Step Synthesis of the Endohedral Stannaspherene [Ir@Sn12]3- via the Capped Cluster Anion [Sn9Ir(cod)]3. Chem. Eur. J. 16, 1793-1798 (2010).
- Gillett-Kunnath, M. M. P. aik, Jensen, J. I., Taylor, S. M., D, &J., Sevov, S. C. Metal-Centered Deltahedral Zintl Ions: Synthesis of [Ni@Sn9]4- by Direct Extraction from Intermetallic Precursors and of the Vertex-Fused Dimer [{Ni@Sn8(μ-Ge)1/2}2]4. Inorg. Chem. 50, 11695-11701 (2011).
- Ugrinov, A., Sevov, S. C. Ph2Bi-(Ge9)-BiPh2]2-: A Deltahedral Zintl Ion Functionalized by Exo-Bonded Ligands. J. Am. Chem. Soc. 124, 2442-2443 (2002).
- Ugrinov, A., Sevov, S. C. Derivatization of Deltahedral Zintl Ions by Nucleophilic Addition: [Ph-Ge9-SbPh2]2- and [Ph2Sb-Ge9-Ge9-SbPh2]4. J. Am. Chem. Soc. 125, 14059-14064 (2003).
- Ugrinov, A., Sevov, S. C. Rationally Functionalized Deltahedral Zintl Ions: Synthesis and Characterization of [Ge9-ER3]3-, [R3E-Ge9-ER3]2-, and [R3E-Ge9-Ge9-ER3]4- (E= Ge, Sn; R = Me, Ph). Chem. Eur. J. 10, 3727-3733 (2004).
- Hull, M., Ugrinov, A., Petrov, I., Sevov, S. C. Alkylation of Deltahedral Zintl Clusters: Synthesis of [R-Ge9-Ge9-R]4- (R = tBu, sBu, nBu, tAm) and Structure of [tBu-Ge9-Ge9-tBu]4. Inorg. Chem. 46, 2704-2708 (2007).
- Hull, M., Sevov, S. C. Addition of Alkenes to Deltahedral Zintl Clusters by Reaction with Alkynes: Synthesis and Structure of [Fc-CH=CH-Ge9-CH=CH-Fc]2-, an Organo-Zintl-Organometallic Anion. Angew. Chem. Int. Ed. 46, 6695-6698 (2007).
- Hull, M., Sevov, S. C. Organo-Zintl Clusters Soluble in Conventional Organic Solvents: Setting the Stage for Organo-Zintl Cluster Chemistry. Inorg. Chem. 46, 10953-10955 (2007).
- Chapman, D. J., Sevov, S. C. Tin-Based Organo-Zintl Ions: Alkylation and Alkenylation of Sn94. Inorg. Chem. 47, 6009-6013 (2008).
- Hull, M., Sevov, S. C. Functionalization of Nine-Atom Deltahedral Zintl Ions with Organic Substituents: Detailed Studies of the Reactions. J. Am. Chem. Soc. 131, 9026-9037 (2009).
- Kocak, F. S., Zavalij, P. Y., Lam, Y. -F., Eichhorn, B. W. Substituent-dependent exchange mechanisms in highly fluxional RSn93- anions. Chem. Commun. , 4197-4199 (2009).
- Gillett-Kunnath, M. M., Petrov, I., Sevov, S. C. Heteroatomic Deltahedral Zintl Ions of Group 14 and their Alkenylation. Inorg. Chem. 48, 721-729 (2010).
- Gillett-Kunnath, M. M., Oliver, A. G., Sevov, S. C. "n-Doping" of Deltahedral Zintl Ions. J. Am. Chem. Soc. 133, 6560-6562 (2011).
- Gaumet, J. J., Strouse, G. F. Electrospray Mass Spectrometry of Semiconductor Nanoclusters: Comparative Analysis of Positive and Negative Ion Mode. J. Am. Soc. Mass. Spectrom. 11, 338-344 (2000).
- Fässler, T. F. Lone Pair Interactions in Zintl Phases: Band Structure and Real Space Analysis of the cP124 Clathrate Structure Type. Z. Anorg. Allg. Chem. 624, 569-577 (1998).
- Guloy, A. M., Ramlau, R., Tang, Z., Schnelle, W., Baitinger, M., Grin, Y. A guest-free germanium clathrate. Nature. 443, 320-323 (2006).
- Guloy, A. M., Tang, Z., Ramlau, R., Böhme, B., Baitinger, M., Grin, Y. Synthesis of the Clathrate-II K8.6(4)Ge136 by Oxidation of K4Ge9 in an Ionic Liquid. Eur. J. Inorg. Chem. 17, 2455-2458 (2009).
- Chandrasekharan, N., Sevov, S. C. Anodic Electrodeposition of Germanium Films from Ethylenediamine Solution of Deltahedral Ge94- Zintl Ions. J. Electrochem. Soc. 157, C140-C145 (2010).
- Zheng, W. J., Thomas, O. C., Lippa, T. P., Xu, S. J., Bowen, K. H. The Ionic KAl13 molecule: A stepping stone to cluster-assembled materials. J. Chem. Pys. 124, 144304-144304 (2006).
- Riley, A. E., Tolbert, S. H. Syntehsis and characterization of tin telluride inorganic/organic composite materials with nanoscale periodicity through solution-phase self-assembly: a new class of composite materials based on Zintl cluster self-oligomerization. Res. Chem. Intermed. 33, 111-124 (2007).
- Sun, D., Riley, A. E., Cadby, A. J., Richman, E. K., Korlann, S. D., Tolbert, S. H. Hexagonal nanoporous germanium through surfactant-driven self-assembly of Zintl Clusters. Nature. 441, 1126-1130 (2006).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved