A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Gold Nanostar Synthesis with a Silver Seed Mediated Growth Method
In This Article
Summary
We synthesized star shaped gold nanostars using a silver seed mediated growth method. The diameter of the nanostars ranges from 200 to 300 nm and the number of tips vary from 7 to 10. The nanoparticles have a broad surface plasmon resonance mode centered in the near infrared.
Abstract
The physical, chemical and optical properties of nano-scale colloids depend on their material composition, size and shape 1-5. There is a great interest in using nano-colloids for photo-thermal ablation, drug delivery and many other biomedical applications 6. Gold is particularly used because of its low toxicity 7-9. A property of metal nano-colloids is that they can have a strong surface plasmon resonance 10. The peak of the surface plasmon resonance mode depends on the structure and composition of the metal nano-colloids. Since the surface plasmon resonance mode is stimulated with light there is a need to have the peak absorbance in the near infrared where biological tissue transmissivity is maximal 11, 12.
We present a method to synthesize star shaped colloidal gold, also known as star shaped nanoparticles 13-15 or nanostars 16. This method is based on a solution containing silver seeds that are used as the nucleating agent for anisotropic growth of gold colloids 17-22. Scanning electron microscopy (SEM) analysis of the resulting gold colloid showed that 70 % of the nanostructures were nanostars. The other 30 % of the particles were amorphous clusters of decahedra and rhomboids. The absorbance peak of the nanostars was detected to be in the near infrared (840 nm). Thus, our method produces gold nanostars suitable for biomedical applications, particularly for photo-thermal ablation.
Protocol
1. Silver seed preparation
- Prepare a stock solution of silver nitrate (AgNO3) by taking an arbitrary mass and mixing it with 10 mL of deionized (DI) water. Calculate molarity of the solution. Keep the solution in a dark place to isolate it from light.
- Add 14.7 mg of sodium citrate tribasic (Na3C6H5O7) to 10 mL of DI water to make a 5 mM solution. Shake the vial until the powder is dissolved.
- Add 15.1 mg of sodium borohydride (NaBH4) to another vial with 10 mL of DI water to make a 40 mM solution. Close the vial immediately. Gently shake the solution by hand and place it in a beaker with ice. Place the beaker in the refrigerator and start a timer (t1 = 0). The freshly made solution will be used in 15 min which is enough time to cool it down.
- From the stock solution of silver nitrate, 1.1), prepare 10 mL at 0.25 mM. Place a stirring magnet in the vial and start stirring.
- Add 0.25 mL of the sodium citrate tribasic solution 1.2) to 1.4).
- At t1 = 15 min remove the sodium borohydride solution, 1.3), from the refrigerator. Using a pipette take 0.4 mL of this solution and add it to 1.5). Note: Add the solution in a single quick stroke. The color will turn to yellow. Stir the solution for 5 min.
- At t1 = 20 min stop stirring, remove the magnet from the vial and keep the vial in a dark place. Do not close the vial.
- Keep the solution in the dark at room temperature for at least 2 hours before using. Preferably use the seeds within one week from preparation.
2. Growth solution preparation
- Prepare 80 mM of ascorbic acid (C6H8O6) by adding 140 mg to 10 mL of DI water.
- Prepare 10 mL of a concentrated solution of gold chloride (HAuCl4). Calculate the molarity of the solution. Keep the solution isolated from light.
- Prepare 20 mL of 50 mM of cetyltrimethylammonium bromide (CTAB - C19H42BrN) by adding 364 mg to a vial with 20 mL of DI water. Immediately place a stirring magnet into the vial and start stirring on a warm plate at 30°C. After CTAB powder is completely dissolved and the solution becomes transparent turn off the heater of the plate but keep stirring through step 2.7).
- Add solution 1.1) to solution 2.3) to obtain a final molarity of 4.9x10-2 mM. Start a timer (t2 = 0).
- At t2 = 1 min add solution 2.2) to 2.4) to obtain a final molarity of 0.25 mM.
- At t2 = 2 min add 0.1 mL of 2.1) to 2.5). The solution will turn colorless.
- At t2 = 2 min 20 sec add 0.05 mL of 1.8) (silver seeds) to 2.6). Stir the suspension for 15 min. The suspension will initially turn blue and then brown.
- At t2 = 17 min stop stirring, remove the magnet and keep the suspension at room temperature for 24 hours.
3. Separating gold nanostars from CTAB for imaging, characterization or experimentation
Note: CTAB may crystallize at room temperature. To dissolve the crystals heat up the gold colloid to 30°C or immerse the vial in hot tap-water until the crystals dissolve.
- Sonicate the suspension for 2 min.
- Centrifuge the suspension for 5 min at 730 rcf. Nanostars will accumulate on the wall of the tube.
- Remove as much of the suspension with a pipette taking care not to remove the nanostars.
- Add DI water to the tube and sonicate for 2 min.
- Centrifuge the suspension for 3 min at 460 rcf. The suspension contains less CTAB, therefore lower centrifugal force is needed to separate the nanostars.
- Repeat steps 3.3) and 3.4).
- Add DI water to the suspension and centrifuge for 3 min at 380 rcf.
- Repeat steps 3.3) and 3.4). The nanostars are ready for imaging, spectroscopy, or experimentation.
4. Representative results:
Figure 1 shows transmission electron microscope (TEM) images of the silver seeds imaged using a JEOL 2010-F TEM. The seeds have a spherical shape and an average size of 15 nm. Gold nanostars are imaged using a Hitachi S-5500 in scanning electron microscope (SEM) mode. Figure 2 shows increasing magnifications of the nanostars synthesized with our method. Star shaped particles are approximately 70 % of all the particles in the colloid. Non-formed stars appear like amorphous clusters of decahedra and rhomboids (not shown). Figure 3 shows several single gold nanostars. The size of the nanostars ranges from 200 nm to 300 nm and the number of tips vary from 7 to 10. If the gold nanoparticles synthesized by this method are left in CTAB they retain their shape for at least 1 month after synthesis.
We measured the absorption spectra of the silver seeds and nanostars using an Olis Cary-14 spectrophotometer. The peak absorption of the seeds was at 400 nm, while the peak absorption of the nanostars was between 800 nm and 850 nm (Figure 4).
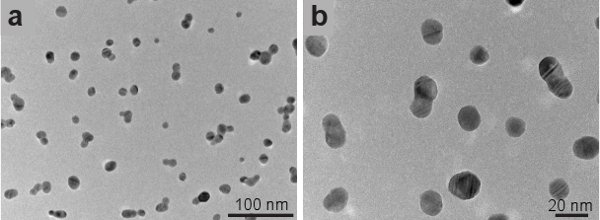
Figure 1. Transmission electron microscope images of silver seeds.

Figure 2. Scanning electron microscope images of gold nanostars.
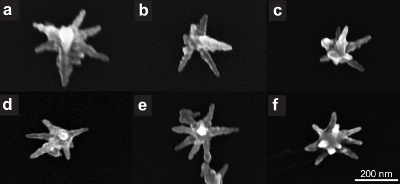
Figure 3. Scanning electron microscope images of single gold nanostars.
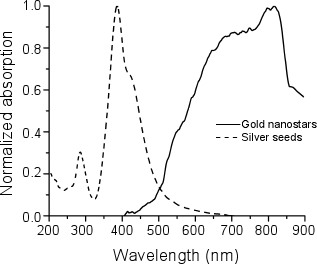
Figure 4. Normalized absorption spectra of silver seeds (dashed line) and gold nanostars (solid line).
Access restricted. Please log in or start a trial to view this content.
Discussion
In this work we have presented a method to synthesize gold nanostars using silver seeds. We found that silver seeds resulted in a yield of 70 % production of nanostars. The nanostars have a near infrared absorption peak, corresponding to their surface plasmon resonance mode, centered between 800 nm and 850 nm 7, 23. These properties properties allow our gold nanostars to be of use for biomedical applications 24-26, such as photo-thermal ablation.
A major difference betwe...
Access restricted. Please log in or start a trial to view this content.
Disclosures
No conflicts of interest declared.
Acknowledgements
This research was supported by the National Science Foundation Partnerships for Research and Education in Materials (PREM) Grant No. DMR-0934218. It was also supported by Award Number 2G12RR013646-11 from the National Center For Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| Sodium citrate tribasic dehydrate | Sigma-Aldrich | S4641 | 99.0 % |
| Silver nitrate | Aldrich | 204390 | 99.9999 % |
| Sodium borohydride | Aldrich | 213462 | 99 % |
| L-Ascorbic acid | Sigma-Aldrich | 255564 | 99+ % |
| Gold chloride trihydrate | Aldrich | 520918 | 99.9+ % |
| Hexadecyltrimethylammonium bromide (CTAB) | Sigma-Aldrich | H6269 | |
| JEOL 2010-F | JEOL | Transmission electron microscope | |
| Hitachi S-5500 | Hitachi | Used in scanning electron microscope mode | |
| Olis Cary-14 spectrophotometer | Olis | Spectrophotometer |
References
- Irimpan, L., Nampoori, V. P. N., Radhakrishnan, P., Krishnan, B., Deepthy, A. Size-dependent enhancement of nonlinear optical properties in nanocolloids of ZnO. Journal of Applied Physics. 103, (2008).
- Sharma, V., Park, K., Srinivasarao, M. Colloidal dispersion of gold nanorods: Historical background, optical properties, seed-mediated synthesis, shape separation and self-assembly. Materials Science and Engineering: R: Reports. 65, 1-38 (2009).
- El-Sayed, M. A. Some interesting properties of metals confined in time and nanometer space of different shapes. Accounts of Chemical Research. 34, 257-2564 (2001).
- Daniel, M. C., Astruc, D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chemical reviews. 104, 293-346 (2004).
- Burda, C., Chen, X., Narayanan, R., El-Sayed, M. A. Chemistry and Properties of Nanocrystals of Different Shapes. Chemical reviews. 105, 1025-1102 (2005).
- Hu, M., Chen, J. Y. X., Li, J. Y., Au, L., Hartland, G. V., Li, X. D., Marquez, M., Xia, Y. N. Gold nanostructures: engineering their plasmonic properties for biomedical applications. Chemical Society Reviews. 35, 1084-1094 (2006).
- Seo, J. T., Yang, Q., Kim, W. J., Heo, J., Ma, S. M., Austin, J., Yun, W. S., Jung, S. S., Han, S. W., Tabibi, B., Temple, D. Optical nonlinearities of Au nanoparticles and Au/Ag coreshells. Opt. Lett. 34, 307-309 (2009).
- Jeong, S., Choi, S. Y., Park, J., Seo, J. -H., Park, J., Cho, K., Joo, S. -W., Lee, S. Y. Low-toxicity chitosan gold nanoparticles for small hairpin RNA delivery in human lung adenocarcinoma cells. Journal of Materials Chemistry. 21, 13853-13859 (2011).
- Huang, X., Jain, P. K., El-Sayed, I. H., El-Sayed, M. A. Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine. 2, 681-693 (2007).
- Link, S., El-Sayed, M. A. Shape and size dependence of radiative, non-radiative and photothermal properties of gold nanocrystals. International Reviews in Physical Chemistry. 19, 409-453 (2000).
- El-Sayed, I. H., Huang, X. H., El-Sayed, M. A. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Letters. 239, 129-135 (2006).
- O'Neal, D. P., Hirsch, L. R., Halas, N. J., Payne, J. D., West, J. L. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Letters. 209, 171-176 (2004).
- Nehl, C. L., Liao, H. W., Hafner, J. H. Optical properties of star-shaped gold nanoparticles. Nano Letters. 6, 683-688 (2006).
- Pazos-Perez, N., Rodriguez-Gonzalez, B., Hilgendorff, M., Giersig, M., Liz-Marzan, L. M. Gold encapsulation of star-shaped FePt nanoparticles. Journal of Materials Chemistry. 20, 61-64 (2010).
- Sahoo, G. P., Bar, H., Bhui, D. K., Sarkar, P., Samanta, S., Pyne, S., Ash, S., Misra, A. Synthesis and photo physical properties of star shaped gold nanoparticles. Colloids and Surfaces A: Physicochemical and Engineering Aspects. , 375-371 (2011).
- Senthil Kumar, P., Pastoriza-Santos, I., Rodriguez-Gonzalez, B., Garcia de Abajo, F. J., Liz-Marzan, L. M. High-yield synthesis and optical response of gold nanostars. Nanotechnology. 19, (2008).
- Goodrich, G. P., Bao, L. L., Gill-Sharp, K., Sang, K. L., Wang, J., Payne, J. D. Photothermal therapy in a murine colon cancer model using near-infrared absorbing gold nanorods. Journal of Biomedical Optics. 15, (2010).
- Zhang, D., Neumann, O., Wang, H., Yuwono, V. M., Barhoumi, A., Perham, M., Hartgerink, J. D., Wittung-Stafshede, P., Halas, N. J. Gold Nanoparticles Can Induce the Formation of Protein-based Aggregates at Physiological pH. Nano Lett. 9, 666-671 (2009).
- Alkilany, A. M., Nagaria, P. K., Hexel, C. R., Shaw, T. J., Murphy, C. J., Wyatt, M. D. Cellular uptake and cytotoxicity of gold nanorods: molecular origin of cytotoxicity and surface effects. Small. 5, 701-708 (2009).
- Sun, L., Liu, D., Wang, Z. Functional gold nanoparticle-peptide complexes as cell-targeting agents. Langmuir. 24, 10293-10297 (2008).
- Park, J., Estrada, A., Sharp, K., Sang, K., Schwartz, J. A., Smith, D. K., Coleman, C., Payne, J. D., Korgel, B. A., Dunn, A. K., Tunnell, J. W. Two-photon-induced photoluminescence imaging of tumors using near-infrared excited gold nanoshells. Opt. Express. 16, 1590-1599 (2008).
- Nikoobakht, B., El-Sayed, M. A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chemistry of Materials. 15, 1957-1962 (2003).
- Hao, F., Nehl, C. L., Hafner, J. H., Nordlander, P. Plasmon resonances of a gold nanostar. Nano Letters. 7, 729-732 (2007).
- Hao, F., Nordlander, P., Sonnefraud, Y., Dorpe, P. V. an, Maier, S. A. Tunability of Subradiant Dipolar and Fano-Type Plasmon Resonances in Metallic Ring/Disk Cavities: Implications for Nanoscale Optical Sensing. ACS Nano. 3, 643-652 (2009).
- Sweeney, C. M., Hasan, W., Nehl, C. L., Odom, T. W. Optical Properties of Anisotropic Core-Shell Pyramidal Particles. Journal of Physical Chemistry A. 113, 4265-4268 (2009).
- Dickerson, E. B., Dreaden, E. C., Huang, X. H., El-Sayed, I. H., Chu, H. H., Pushpanketh, S., McDonald, J. F., El-Sayed, M. A. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Letters. 269, 57-66 (2008).
- Jana, N. R., Gearheart, L., Murphy, C. J. Wet chemical synthesis of high aspect ratio cylindrical gold nanorods. Journal of Physical Chemistry B. 105, 4065-4067 (2001).
- Jana, N. R., Gearheart, L., Murphy, C. J. Seed-mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant template. Advanced Materials. 13, 1389-1393 (2001).
- Xiao, J., Qi, L. Surfactant-assisted, shape-controlled synthesis of gold nanocrystals. Nanoscale. 3, 1383-1396 (2011).
- Tao, A. R., Habas, S., Yang, P. Shape control of colloidal metal nanocrystals. Small. 4, 310-325 (2008).
- Cole, J. R., Mirin, N. A., Knight, M. W., Goodrich, G. P., Halas, N. J. Photothermal Efficiencies of Nanoshells and Nanorods for Clinical Therapeutic Applications. Journal of Physical Chemistry C. 113, 12090-12094 (2009).
- Choi, J. S., Park, J. C., Nah, H., Woo, S., Oh, J., Kim, K. M., Cheon, G. J., Chang, Y., Yoo, J., Cheon, J. A hybrid nanoparticle probe for dual-modality positron emission tomography and magnetic resonance imaging. Angew. Chem. Int. Ed. Engl. 47, 6259-6262 (2008).
- Chithrani, B. D., Ghazani, A. A., Chan, W. C. W. Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells. Nano Letters. 6, 662-668 (2006).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved