A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Reproducible Mouse Sciatic Nerve Crush and Subsequent Assessment of Regeneration by Whole Mount Muscle Analysis
In This Article
Summary
In this report we describe a method to crush mouse sciatic nerve. This method uses readily available hemostatic forceps and easily and reproducibly produces complete sciatic nerve crush. In addition, we describe a method to prepare muscle whole mounts suitable for analysis of nerve regeneration after sciatic nerve crush.
Abstract
Regeneration in the peripheral nervous system (PNS) is widely studied both for its relevance to human disease and to understand the robust regenerative response mounted by PNS neurons thereby possibly illuminating the failures of CNS regeneration1. Sciatic nerve crush (axonotmesis) is one of the most common models of peripheral nerve injury in rodents2. Crushing interrupts all axons but Schwann cell basal laminae are preserved so that regeneration is optimal3,4. This allows the investigator to study precisely the ability of a growing axon to interact with both the Schwann cell and basal laminae4. Rats have generally been the preferred animal models for experimental nerve crush. They are widely available and their lesioned sciatic nerve provides a reasonable approximation of human nerve lesions5,4. Though smaller in size than rat nerve, the mouse nerve has many similar qualities. Most importantly though, mouse models are increasingly valuable because of the wide availability of transgenic lines now allows for a detailed dissection of the individual molecules critical for nerve regeneration6, 7. Prior investigators have used multiple methods to produce a nerve crush or injury including simple angled forceps, chilled forceps, hemostatic forceps, vascular clamps, and investigator-designed clamps8,9,10,11,12. Investigators have also used various methods of marking the injury site including suture, carbon particles and fluorescent beads13,14,1. We describe our method to obtain a reproducibly complete sciatic nerve crush with accurate and persistent marking of the crush-site using a fine hemostatic forceps and subsequent carbon crush-site marking. As part of our description of the sciatic nerve crush procedure we have also included a relatively simple method of muscle whole mount we use to subsequently quantify regeneration.
Protocol
1. Animal Subjects
1.1. Treatment
- All animal procedures should be performed with the approval of the local Institution's Animal Care and Ethics Committee and in accordance with the Use and Committee and National Institutes of Health guidelines, with measures taken to minimize pain and discomfort.
- Our mice were housed under temperature-controlled conditions on a 12-hour reverse light and dark cycle, while fed mouse chow and water ad libitum.
- For studies of adult regeneration, mice should be at least 6 weeks of age when the sciatic nerve crush is performed. This age is beyond the time at which pruning of polyneural neuromuscular junctions take place.
- In these experiments, we used 6-8 week old C57BL/6 mice acquired from Charles River. When comparing regeneration after sciatic nerve crush (i.e.: rate of axon growth) mouse strains should be the same, as differences in axonal regeneration have been noted between different inbred strains15,16. If genetically manipulated mice are to be used, littermate controls are most appropriate.
1.2. Surgical Preparation
- Animals are deeply anesthetized for surgery using a cocktail of ketamine (100 mg/kg) and xylazine (10 mg/kg) via intraperitoneal injection. Each animal also receives a subcutaneous injection of meloxicam (10 mg/kg) to minimize post-operative pain.
- Both hindquarters are carefully shaved using a surgical clippers (Roboz, RC-5903) and depilation is completed with Nair hair removal cream (found at local pharmacy).
- Skin is cleansed using sterile cotton tipped applicators and betadine surgical scrub (Fisher Scientific, 19066452).
- Ophthalmic ointment (Fisher Scientific, 19082795) is applied to the eyes using sterile cotton tipped applicators.
- The mouse is placed on a clean stainless steel plate, under which has been placed a pre-heated homeothermic blanket system (Harvard Apparatus, 507222F). Animal temperature is maintained at 37°C.
- All limbs are taped down, with care taken to position the hind limbs symmetrically so that the knee joint makes a right angle with the body (Figure 1, panel A).
- The surgical field is covered with a sterile drape. All instruments are sterilized by autoclave or hot bead sterilization (Fine Science Tools, 18000-45) and the surgeon wears a mask, gown, and sterile gloves.
2. Reproducible Sciatic Nerve Crush
- After preparation, a semi-circular incision across midline (Figure 1) is made in the skin. The skin is gently dissected from the underlying musculature, and folded over to remain out of the way during the procedure. It is kept moist using applications of 0.1 mL sterile saline (Hospira, 0409-4888-20) during the procedure.
- Opening the fascial plane between the gluteus maximus and the anterior head of the biceps femoris reveals the sciatic nerve (Figure 1, panel A). For a surgical control, the contralateral sciatic nerve should be exposed and mobilized, but left intact. The gluteal musculature is then re-opposed and sutured using a 6-0 braided silk, non-absorbable sutures (Roboz, SUT-1073-11).
- The experimental sciatic nerve is then exposed in the same fashion, with retractors in place to ease visualization (Figure 1, panel B). The retractors are sterilized prior to use.
Note: although retractor systems are commercially available, they are often quite expensive. We were able to make a satisfactory retractor system using inexpensive hardware supplies and insect pins (see Materials section). - The sciatic nerve is then gently freed from the surrounding connective tissue using iridectomy scissors.
- Using a fine 5/45 (Fine Science Tools, 11251-35) forceps, the nerve is placed on the bottom jaw of a super-fine hemostatic forceps (Fine Science Tools, 13020-12). The three fascicles are sequentially aligned, not on top of each other (Figure 1, inset B). The hemostatic forceps have been engraved with a mark at 1.5 mm from their tip. The outermost portion of the sciatic is placed in line with this mark before crush. This ensures a crush of uniform width, and that the nerve does not extend beyond the jaws of the hemostatic forceps when flattened due to the crushing force. If the nerve extends beyond the forceps tip the nerve will only be partially crushed.
- The crush is made perpendicular to the nerve at 45 mm from the third toe, as measured by a thread that approximates the path of the sciatic nerve. The nerve is crushed once for 15 seconds at 3 clicks of the hemostatic forceps. Care is taken not to stretch the nerve. When the hemostats are re-opened, the entire nerve should be translucent at the crush site.
- A second pair of hemostatic forceps (identical to the first) that has been pre-dipped in powdered carbon (Fisher Scientific, C272-500) is used to mark the crush site. The nerve is crushed at the same crush site for 15 seconds at 3 clicks. No carbon marking should extend beyond the boundary of the initial crush. This is particularly important if precise marking of the crush site is required. Prior to use, carbon powder is sterilized by exposure to UV light for two hours, and thereafter is handled using sterile technique.
- To pre-dip the forceps in carbon but preclude widespread carbon at the surgical site, the forceps are opened in powdered carbon, then gently closed (but not clicked) shut, and the carbon on the outside of the hemostats is wiped off using sterile gauze. The forceps are checked under at least 3x magnification to verify that the crushing surfaces are evenly coated in powdered carbon. If necessary, they are re-dipped and wiped.
- The gluteal musculature is re-opposed and sutured in the same way as the contralateral side.
- Finally, the skin incision is closed using 9 mm reflex clips (World Precision Instruments, 500346; Applier: 500345). If 9 mm reflex clips are found to restrict movement, smaller reflex clips or 6-0 sutures (Roboz, SUT-1073-11) can be used instead.
3. Post-Operative Care
- Following the procedure, animals are placed on a heating pad at 37°C until they show signs of movement.
- They are then moved back to their home cage, where water and food are readily accessible on the floor in the form of Hydrogel and wetted food.
4. Semi-Thin Preparation
- Following an overdose of pentobarbital (300 mg/kg) the leg musculature is removed to expose the sciatic nerve*. With the nerve remaining in situ, the hindquarter is immersed in 2% paraformaldehyde and 2% gluteraldehyde in 0.1 M phosphate buffer on ice for 30 minutes.
*If completing whole mount muscle preparation, muscles are harvested before exposing the sciatic nerve. - The nerve is carefully removed, with care taken to only handle the proximal end. Then the nerve is post-fixed in the same fixative for three additional hours.
- After fixation the nerve is rinsed three times in 0.1 M phosphate buffer.
- The nerve is immersed in 2% osmium tetroxide in 0.1 M phosphate buffer for one hour.
- The nerve is then dehydrated by sequential immersion in increasingly concentrated ethanol (50%, 70%, 80%, 95%, 100%, 100%, 100%). Each immersion is 15 minutes.
- The dehydrated nerve is incubated twice in propylene oxide for three minutes each.
- Then the nerve is immersed in a 1:1 mixture of propylene oxide and Embed 812 for at least 6 hours (usually overnight).
- Then the nerve is immersed in a 2:1 mixture of propylene oxide and Embed 812 overnight.
- Finally, the nerve is immersed in pure Embed 812 for six hours, then embedded in the appropriate mold and baked at 60°C for 48 hours.
- 1.0 μm sections are cut from the distal nerve stump at a set distance from the crush site using an Ultracut UCT ultramicrotome (Leica) with a glass knife and stained with toluidine blue. Thin sections may also be produced for examination of ultrastructure by electron microscopy.
5. Whole Mount Muscle Preparation
- First mice are sacrificed with an overdose of pentobarbital (300 mg/kg) and the hind limbs are removed at the knees.
- Four muscles in the hind limb are removed for analysis: the tibialis anterior (TA), extensor digitorum longus (EDL), soleus, and peroneus longus.
- All muscles are removed via careful dissection and pinned through their connective tissue to a black sylgard (Fisher Scientific, NC9492579) coated dish. They are rinsed in PBS, and then fixed in 4% paraformaldehyde for 30 minutes.
- The TA is then rinsed in PBS, embedded in O.C.T (Fisher Scientific, 14-373-65), and quickly frozen in a bath of acetone and dry ice.
- It is stored at -80 °C for thin sectioning in case whole mount preparation fails. In general, the TA is too thick for adequate whole mounts.
- The EDL, soleus, and peroneus longus are rinsed for 3 x 10 minutes in PBS, placed in 0.1 M glycine* (Fisher Scientific, AC12007-0010; diluted in PBS) for 30 minutes, and rinsed 3 x 10 minutes in PBS again. They are then quenched in ice cold 100% methanol for exactly 5 minutes at -20°C, rinsed 3 x 10 minutes in PBS, and bathed in fluorescently conjugated alpha-bungarotoxin (diluted 1:200 in PBS) for 30 minutes. The muscles are rinsed for 3 x 10 minutes in PBS, followed by a 1-hour block in 2 % BSA (KPL, 50-61-00) and 0.2 % Triton X-100 (Dot Scientific Incorporated, 9002-93-1) in PBS*, and incubated overnight while rocking at 4 °C in a cocktail of primary antibodies diluted in the same 2% BSA/0.2% Triton block.
*Glycine and blocking solutions are made on the same day as muscle harvesting and stirred for 30-60 minutes at room temperature prior to use.- To mark axons and neuromuscular synapses, we use a combination of a mouse monoclonal neurofilament marker (Covance, SMI-312R; diluted 1:1000), a mouse monoclonal synaptic vesicle marker ( SV2 DSHB, diluted 1:1000), and rhodamine conjugated alpha-bungarotoxin (Sigma- Aldrich, diluted 1:200). To mark reactive Schwann cells we use rabbit anti-GAP-43 (Novus Biologicals, NB300-143, diluted 1/500). The primary antibodies are visualized with an IgG1 subtype specific fluorescein conjugated goat anti-mouse secondary antibody and DyLight 649 conjugated donkey anti-rabbit (Jackson ImmunoResearch, diluted 1:200).
- The following day, the muscles are rinsed in PBS for 3 x 10 minutes, and incubated in secondary antibodies diluted 1:200 (in 2% BSA/0.2% Triton blocking solution). They are then rinsed 2 x 10 minutes in PBS, followed by a 5-minute immersion in DAPI (Invitrogen, D3571; diluted to 300 nM in deionized water), and another 10-minute rinse in PBS.
- Each muscle is then dissected on a black sylgard (Fisher Scientific, NC9492579) coated petri dish. Initially the tendons are removed at their insertion points into the muscle, and then the muscles are thinned by peeling away interior muscle fibers (Figure 3, panels A-C). Care is taken to preserve exterior surface of the muscle, including the endplate bands (Figure 3, panels D-F). The resulting muscles are mounted on plus slides (Fisher Scientific, 12-550-15) with vectashield (Vector Laboratories, H-1000) and 22 x 40 mm glass coverslips (Fisher Scientific, 12-548-5C) sealed by clear nail polish on two sides. When properly mounted on the slide the endplate band contacts the coverslip.
6. Measuring Regeneration
- Regeneration may be estimated in all three muscles (peroneus, EDL, and soleus). We often examine regeneration fourteen days after nerve crush, a time at which a substantial percentage of NMJs are re-innervated. Both earlier and later time points are also suitable, depending upon the scientific question being asked. In each muscle, re-innervation of the entire endplate band is examined. Only en face surface NMJs are scored. In this fashion, at least 200 NMJs per muscle may be quickly examined.
- To score muscle re-innervation we determine a "re-innervation ratio". The denominator is the number of denervated neuromuscular junctions as labeled by α-bungarotoxin binding and Schwann cell GAP-43 immunoreactivity. The numerator is the number of re-innervated NMJs as labeled by neurofilament/SV2 immunoreactivity. If needed, NMJs may be classified as either partially (incomplete coverage of α-bungarotoxin by SV2) or fully (complete coverage of α-bungarotoxin by SV2) re-innervated. For examples of re-innervated and denervated NMJs see Figure 3, panels G and H.
7. Representative Results
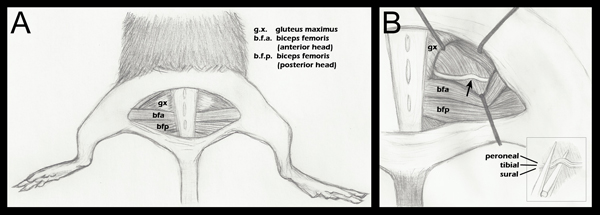
Figure 1. Schematic of hind limb anatomy important to nerve crush. A. A semi-circular skin incision has been made revealing the underlying musculature. B. The gluteal musculature has been separated, and the sciatic nerve revealed (step 2.4 above). An arrow denotes the approximate crush site. Retractor placement is shown as a general guide, and is adjusted during each surgery to ease visualization of the sciatic and approach of the hemostatic forceps for crush. B (inset): Placement of the sciatic on the lower jaw of the hemostatic forceps just before crush (step 2.5 above). Separate fascicles are labeled to demonstrate that they are adjacent horizontally, but not vertically, during the crush. Although three fascicles are labeled in this diagram, one may also see a fourth fascicle, the articular branch of the peroneal. For a more detailed anatomical drawing of the branching patterns of the sciatic nerve fascicles distal to the crush point, please refer to Greene's Anatomy of the Rat, Figure 18817.
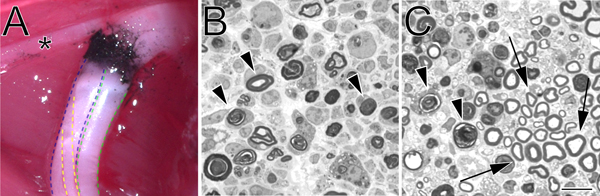
Figure 2. A carbon-marked crush site in situ and toluidine blue-stained, semi-thin sections of crushed sciatic nerve. A. An example of a crush site in situ (left hind limb). Black carbon denotes the crush site. The asterisk marks a branch of the tibial nerve that innervates thigh musculature and serves as a useful landmark during crush surgery. The tibial division of the sciatic nerve is outlined in blue, the peroneal in green, and the sural in yellow. B. Semi-thin sections demonstrating a complete crush performed with hemostatic forceps. C. Semi-thin sections demonstrating an incomplete crush performed with angled forceps. In both images degenerating myelin profiles are marked with arrowheads. In panel C arrows mark examples of preserved myelin profiles and a cluster of spared axons. Scale bar is 10 μm.
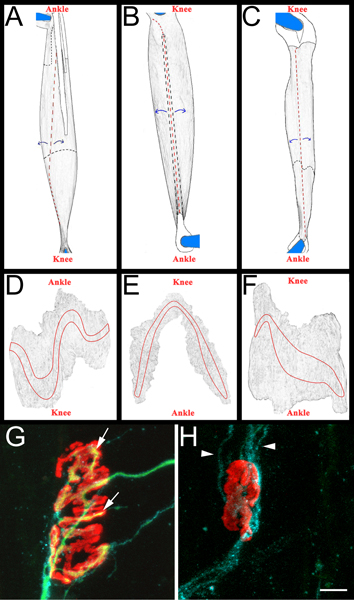
Figure 3. Whole mount muscle schematic and representative NMJs. A-C: Rendered images of EDL (A), peroneus longus (B), and soleus (C) muscles after removal from hind limb. Muscles shown are from right hind limb. Knee and ankle anatomical orientation are also included for reference. Tendons are colored white and outlined in a solid black line when positioned on top of the muscle. They are outlined in a black dashed line when they extend beneath the muscle. Cuts are shown in red dashed lines. Muscle is peeled away from cut sites, as indicated by blue arrows, and subsequently thinned. After thinning, the muscle is separated from the pins by cutting around what remains of the pinned tendons. D-F: Rendered images from whole mount muscles after removal of connective tissue and subsequent thinning. The endplate bands are outlined on each muscle. G-H: Soleus whole mount muscle fourteen days after sciatic nerve crush demonstrating re-innervated (G) and denervated (H) neuromuscular junctions. In these panels, NMJs, axons, and Schwann cells are visualized as described above in section 5.5.1. Acetylcholine receptors are red, axons green, and Schwann cell processes blue. In panel G, the arrows indicate areas of the NMJ that have been re-innervated by an axon. In panel H arrowheads indicate GAP-43 positive Schwann cell processes. Note the absence of axons. Scale bar is 10 μm.
Access restricted. Please log in or start a trial to view this content.
Discussion
We have presented a method to obtain a reliably complete sciatic nerve crush with precise marking of the crush site. As previously mentioned, sciatic nerve crush is a common model of peripheral nerve injury in mice and rats. Although each method of crush has its advantages and disadvantages, we found this method produced a complete crush that was easily marked with a minimum of special equipment (e.g. special clamps, etc).
Crush Methods
The most common instrument use...
Access restricted. Please log in or start a trial to view this content.
Disclosures
We have nothing to disclose.
Acknowledgements
This work was supported by the NIH grants K08NS065157 (to T.A.F.) In addition, the Penn Center for Musculoskeletal Disorders, Award Number P30AR050950 from the National Institute of Arthritis, Musculoskeletal and Skin Diseases supported this work (T.A.F. and Steven S. Scherer). Finally, Shriners Pediatric Research Center Seed funding (T.A.F) supported this work. We would like to acknowledge Dr. Young-Jin Son for initially demonstrating the whole mount procedure and Amy A. Kim for her assistance in producing the sketches of Figure 1.
Access restricted. Please log in or start a trial to view this content.
References
- Pan, Y. A., Misgeld, T., Lichtman, J. W., Sanes, J. R. Effects of neurotoxic and neuroprotective agents on peripheral nerve regeneration assayed by time-lapse imaging in vivo. J. Neurosci. 23, 11479-11488 (2003).
- Magill, C., Tong, A., Kawamura, D., Hayashi, A., Hunter, D. Reinnervation of the tibialis anterior following sciatic nerve crush injury: A confocal microscopic study in transgenic mice. Exp. Neurol. 207, 64-74 (2007).
- Amado, S., Simñes, M. J., Armada-da-Silva, P. A. S., Luís, A. L., Shirosaki, Y. Use of hybrid chitosan membranes and N1E-115 cells for promoting nerve regeneration in an axonotmesis rat model. Biomaterials. 29, 4409-4419 (2008).
- Luís, A. L., Rodrigues, J. M., Geuna, S., Amado, S., Simðes, M. J. Neural cell transplantation effects on sciatic nerve regeneration after a standardized crush injury in the rat. Microsurgery. 28, 458-470 (2008).
- Luís, A. L., Amado, S., Geuna, S., Rodrigues, J. M., Simðes, M. J. Long-term functional and morphological assessment of a standardized rat sciatic nerve crush injury with a non-serrated clamp. J. Neurosci. Methods. 163, 92-104 (2007).
- Baptista, A. F., de Souza Gomes, J. R., Oliveira, J. T., Santos, S. M. G., Vannier-Santos, M. A. A new approach to assess function after sciatic nerve lesion in the mouse - adaptation of the sciatic static index. J. Neurosci. Methods. 161, 259-264 (2007).
- Ronchi, G., Raimondo, S., Varejão, A. S. P., Tos, P., Perroteau, I. Standardized crush injury of the mouse median nerve. J. Neurosci. Methods. 188, 71-75 (2010).
- Berg, A., Zelano, J., Cullheim, S. Netrin G-2 ligand mRNA is downregulated in spinal motoneurons after sciatic nerve lesion. Neuroreport. 21, 782-785 (2010).
- Girolami, E. I., Bouhy, D., Haber, M., Johnson, H., David, S. Differential expression and potential role of SOCS1 and SOCS3 in Wallerian degeneration in injured peripheral nerve. Exp. Neurol. 223, 173-182 (2010).
- Hossain-Ibrahim, M. K., Rezajooi, K., Stallcup, W. B., Lieberman, A. R., Anderson, P. N. Analysis of axonal regeneration in the central and peripheral nervous systems of the NG2-deficient mouse. BMC Neurosci. 8, 80-80 (2007).
- Thornton, M. R., Mantovani, C., Birchall, M. A., Terenghi, G. Quantification of N-CAM and N-cadherin expression in axotomized and crushed rat sciatic nerve. J. Anat. 206, 69-78 (2005).
- Beer, G. M., Steurer, J., Meyer, V. E. Standardizing nerve crushes with a non-serrated clamp. J. Reconstr. Microsurg. 17, 531-534 (2001).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved