Method Article
Parasite Induced Genetically Driven Autoimmune Chagas Heart Disease in the Chicken Model
In This Article
Summary
The inoculation of Trypanosoma cruzi in fertile eggs prior to incubation renders the parasite kDNA minicircle integration in embryo cells genome. Crossbreeding reveals the vertical transfer of the mutations to progeny. The kDNA integrates into coding regions at several chromosomes and the chickens die with an inflammatory autoimmune heart disease.
Abstract
The Trypanosoma cruzi acute infections acquired in infancy and childhood seem asymptomatic, but approximately one third of the chronically infected cases show Chagas disease up to three decades or later. Autoimmunity and parasite persistence are competing theories to explain the pathogenesis of Chagas disease 1, 2. To separate roles played by parasite persistence and autoimmunity in Chagas disease we inoculate the T. cruzi in the air chamber of fertilized eggs. The mature chicken immune system is a tight biological barrier against T. cruzi and the infection is eradicated upon development of its immune system by the end of the first week of growth 3. The chicks are parasite-free at hatching, but they retain integrated parasite mitochondrial kinetoplast DNA (kDNA) minicircle within their genome that are transferred to their progeny. Documentation of the kDNA minicircle integration in the chicken genome was obtained by a targeted prime TAIL-PCR, Southern hybridizations, cloning, and sequencing 3, 4. The kDNA minicircle integrations rupture open reading frames for transcription and immune system factors, phosphatase (GTPase), adenylate cyclase and phosphorylases (PKC, NF-Kappa B activator, PI-3K) associated with cell physiology, growth, and differentiation 3, 5-7, and other gene functions. Severe myocarditis due to rejection of target heart fibers by effectors cytotoxic lymphocytes is seen in the kDNA mutated chickens, showing an inflammatory cardiomyopathy similar to that seen in human Chagas disease. Notably, heart failure and skeletal muscle weakness are present in adult chickens with kDNA rupture of the dystrophin gene in chromosome 1 8. Similar genotipic alterations are associated with tissue destruction carried out by effectors CD45+, CD8γδ+, CD8α lymphocytes. Thus this protozoan infection can induce genetically driven autoimmune disease.
Protocol
1. Growth of Parasites
- Grow trypomastigote forms of T. cruzi Berenice and the β-galactosidase-expressing Tulahuen T. cruzi MHOM/CH/00 C4 in murine muscle cell (L6) cultivated in Dulbecco minimal essential medium with 10% FSB, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 250 nM L-glutamin (pH 7.2), 5% CO2 at 37 °C. The free-swimming trypomastigotes in the supernatant medium were used to inoculate chicken eggs.
- Grow Leishmania braziliensis (Lb) LTB300 stock grown in DMEM with 20% FBS. The Lb promastigote form in the exponential growth phase was used to inoculate eggs 9.
2. Parasite Inoculation in Fertilized Chicken Eggs
- Inoculate a suspension of 100 T. cruzi trypomastigotes in 10 μl of culture medium through a 2-mm diameter hole into the egg shell on top of the air chamber of stage X fertile eggs. The invasion and replication of the virulent parasites into the embryo cells are shown in Video S1. The control groups are as follows: a) control chickens; b) mock control eggs receiving 10 μl of culture medium; c) Stage X fertile eggs inoculated with a suspension of 100 Lb promastigotes in 10 μl of culture medium. T. cruzi and Lb belong to the kinetoplastid family. Respectively, these protozoans grow free in the cytoplasm or in parasitophorous vacuole of host cells 10.
- Seal holes with adhesive tape.
- Incubate the T. cruzi-infected eggs and mock and uninfected control samples at 37.5 °C and 65% humidity for 21 days.
- Keep the chicks that hatch in the incubator for 24 h and thereafter at 32 °C for three weeks.
3. Obtaining Samples for DNA Extraction
- Peripheral blood mononuclear cells were obtained from chickens: a) hatched from T. cruzi inoculated eggs; b) controls; c) mocks receiving 10 μl of culture medium; d) hatched from Lb inoculated eggs; White blood cells from chickens are processed for DNA extraction according with a standard protocol 11.
- Extract DNA also from semen collected from roosters, and from unfertile oocytes (< 5 mm) collected from hens hatched from eggs inoculated with T. cruzi, and from hens hatched from control eggs 3, 4.
- Extract kDNA from the T. cruzi epimastigote forms and, also, from Lb promastigotes, as described elsewhere 9.
4. Primers and Probes Used
The primers used for PCR amplifications and the thermal conditions are shown in Table 1.
The probes used in Southern blot hybridizations were:
- Wild-type minicircle (~1.4 kb) sequences purified from T. cruzi epimastigote forms;
- Minicircle fragments (362 bp) obtained by NsiI digests of wild-type kDNA;
- Nuclear DNA (nDNA) repetitive sequence (188 bp) obtained by amplification of the parasite DNA with the Tcz1/2 primers. The probes were purified from 1% agarose gels 3.
- Wild-type minicircle (~0.820 kb) sequences from Lb promastigotes.
5. PCR Analyses
- Run standard PCR procedure with genomic DNAs from infected chicks and uninfected controls and mock using T. cruzi nDNA Tcz1/2 12 and kDNA s35/s36 13 primers. Also, run PCR with genomic DNAs from chickens hatched from Lb infected-eggs using the protozoan specific Lb3 and Lb5 primers (Table 1).
- Make reaction mix with 100 ng template DNA, 0.4 μM of each pair of primers, 2 U Taq DNA polymerase, 0.2 mM dNTP, and 1.5 mM MgCl2 in a 25 μL final volume.
- Set thermocycler program for 95 °C for 5 min, 30 cycles of 30 sec at 95 °C/30 sec at 68 °C/1 min at 72 °C with 5 min final extension before refrigeration.
- Analyze the amplification products in 1.3% agarose gel, which is transferred to a positively-charged nylon membrane (GE Life Sciences) by the alkaline method for hybridization with specific probes labeled with [α-32P] dATP using Random Primer Labeling Kit (Invitrogen, Carlsbad, CA).
6. Genomic Southern Blots
- Use MboI and/or with EcoRI (Invitrogen) enzymes that make single cuts in minicircles integrated into DNA samples of body tissues.
- Digest DNA from uninfected control chickens and from chickens hatched from eggs inoculated with virulent T. cruzi forms.
- Subject the digests of DNA from T. cruzi and from chicken test samples to electrophoresis in 0.8% agarose gel at 50 V overnight at 4 °C.
- Transfer separated DNA bands to positively charged nylon membrane.
- Hybridize the DNA bands with radio labeled kDNA probe.
- Wash the membrane twice for 15 min at 65 °C with 2X SSC and 0.1% SDS, twice for 15 min at 65 °C each with 0.2X SSC and 0.1% SDS, and autoradiograph for variable periods of time.
7. Targeted Prime TAIL-PCR
- Obtain amplification of the kDNA minicircle integrated into the chicken genome by a modified TAIL-PCR technique, which combines kDNA primers with specific primer sets 2 in three walk-in cycles nested PCR, as shown in Figure 1.
- Primary cycle: Each reaction includes 200 ng template DNA, 2.5 mM MgCl2, and 0.4 μM of kDNA primers (S34 or S67), 0.2 mM dNTPs, 2.5 U Taq Platinum (Invitrogen, Carlsbad, CA). Use the kDNA primers in combination with 0.04 μM of Gg primers (Gg1 to Gg6, Table 1), separately. Set temperatures from 57.9 to 60.1 °C for kDNA primers and from 59.9 to 65.6 °C for CR-1 primer. Note that these temperatures are higher than those (~45 °C) required for the arbitrary degenerated primers used in the TAIL-PCR 10. Use temperature and cycles (MyCycle Thermocycler, Bio-Rad Laboratories, Hercules, CA) described in a previous paper 3.
- Secondary cycle: Dilute PCR products from primary cycle 1:40 (v/v) in water. kDNA primers S35 and S35 antisense replaced the previous ones, along with the same Gg primers.
- Tertiary cycle: Dilute PCR products from secondary cycle 1:10 (v/v) in water and combine Gg primers with S67 antisense or S36, separately.
- Clone the PCR tertiary cycle products: Clone directly in pGEM T easy vector (Promega, Madison, WI) the products of last amplification that hybridize with kDNA probe.
- Select clones by hybridization with kDNA probe and sequence.
- Validate the tpTAIL-PCR in a mix of 300 pg of kDNA from T. cruzi with 200 ng of DNA from control birds never exposed to kDNA. The temperature and amplification cycles are the same used for the test birds' DNA.
8. Chagas Disease Clinic Manifestation
- Monitor growth and development of chickens hatched from T. cruzi infected eggs and of healthy controls hatched from non-infected eggs daily for mortality and weekly for disease manifestations.
- Detect clinical abnormalities in those chickens (Figure 2) and make electrocardiograph (ECG) recordings to evaluate the electrical axes, heart rates and arrhythmias 3.
- Subject kDNA-mutated and controls chickens monthly to ECG recordings of augmented ventricular unipolar leads aVF (left leg), aVL (left arm), and aVR (right arm), and to assess deviation of mean electrical axis to the left, which is suggestive of heart enlargement 3.
9. Pathology and Immunochemical Analyses
- Record heart and body weight indexes after natural deaths of kDNA mutated chickens (Figure 3). Obtain indexes also for control chickens of the same age and gender.
- Take sections from the heart, esophagus, intestines, skeletal muscle, lungs, liver, and kidneys.
- Fix tissue in buffered 10% formalin (pH 7.4), embed in paraffin and cut to 4 μm thick sections for Hematoxylin-Eosin (HE) staining and histological analyses (Figure 3).
- Harvest and bisect tissues from embryos hatched from eggs inoculated with parasites expressing β-galactosidase, and subject to X-Gal-stain.9
- Fix the other half of the embryo tissues in 10% formalin, pH 7.4 and proceed as in step 9.3.
- Cut 4μm thin paraffin embedded tissue section and mount on glass slide for microscopic examination.
- Incubate sections showing X-Gal-stained blue cells with human chagasic antiserum (1:1024 dilution) against anti-T. cruzi antigen.
- Wash sections trice with PBS, pH 7.4, 5 min each.
- Stain blue cells in the embryo tissues by second incubation with a fluorescein-conjugated rabbit anti-human IgG.
- Wash sections with PBS (step 8), mount with coverslip and observe the blue cells light-up green upon examination under UV light at 502 nm wavelength, 200x magnification, for colocalizing T. cruzi in embryo cells.
10. Phenotype Immune System Cells in Heart Lesions
- Phenotype immune effectors cells in tissue sections of heart from kDNA-positive and from control kDNA-negative chickens.
- Place the slides with tissue section embedded in paraffin at 65 °C for 30 min to melt wax previous to submission in four washes in 100% to 70% xylene and then in absolute ethanol PBS for 5 min each.
- Rinse the slides in distilled water, air dry, and treat with specific monoclonal
antibodies (fluorescein- or R-phycoerythrin-conjugated monoclonal antibodies) obtained from SouthernBiotech, Birmingham, AL.
- Use mouse anti-chicken Bu-1 (Bu-1a and Bu-1b alleles, Mr 70-75 kDa) Mab AV20 to recognize monomorphic determinant on the B cell antigens of inbred chickens.
- Use mouse anti-chicken CD45, Ig isotype IgM1 κ specific to chicken thymus lineage cells (Mr 190 to 215-KDa variant).
- Use mouse anti-chicken TCRγδ+ (Mr 90-kDa heterodimer) Mab specific to thymus dependent CD8α+T cells.
- Use mouse anti-chicken Mab CD-8 specific to chicken α chain (Mr 34 kDa) recognize the CD8 cells in thymocytes, spleen, heart, and other tissues.
- Use mouse anti-chicken KuL01 to exclusively recognize monocytes/macrophages of the phagocyte system.
- Wash the slide three times with 0.1 M PBS, pH 7.4, 5 min each after incubation with specific anti-phenotype antibody for 90 min in a moist chamber.
- Assemble the slide with buffered glycerin for exam under a fluorescent light microscope with emission filter of wavelength 567 and 502 nm, respectively, to detect red and green fluorescence-labeled cells (Figure 4).
11. Data Analyses
- Use the chicken genome database (http://www.ncbi.nlm.nih.gov/genome/seq/BlastGen/BlastGen.cgi?taxid=9031) for BLASTn sequence analyses.
- Use CLUSTALW alignments to determine e-value scores.
- Employ the GIRI repeat masking algorithm CENSOR (http://girinst.org/censor/index.php) for localization of different classes of repeats in chimeric sequences.
- Employ the Kinetoplastid Insertion and Deletion Sequence Search Tool (KISS) to identify potential gRNAs in the kDNA sequences, with the aid of WU-Blastn-modified-matrix 3.
- Use T. cruzi sequences http://www.biomedcentral.com/content/supplementary/1471- 2164-8-133-s1.fas to search-in gRNAs in the kDNA-host DNA chimeras. 11.6) Use Student's t and the Kolmorov-Smirnov tests, respectively, to detect significant differences between deviations of electric axes and between heart/body weight indexes obtained in the experimental and control groups, and to detect mortality ratios significant differences between groups of chickens hatched from T. cruzi inoculated eggs and from controls.
12. Representative Results
The inoculation of 100 virulent T. cruzi trypomastigotes into the air chamber of fertile chicken eggs does not reduce significantly the ratios of chicks hatched alive. Approximately 60% hatch healthy chicks and 40% may undergo embryo liquefaction or embryo death at hatching. The surviving chicks retain the kDNA minicircle sequence integrated in the genome. However, it is expected that some chicks will die with cardiomegaly and failure in the weeks after hatching. The remaining chicks will grow to outwardly healthy adults. At all stages of life the DNA extracted from their blood mononuclear cells will yield PCR amplification of kDNA, but not nDNA. The targeted-prime TAIL-PCR 3, 4 products that are cloned and sequence will show the kDNA minicircles integrated mainly in coding regions of macrochromosomes 1 to 5. The chickens showing multiple kDNA integrations into genes coding for cell growth and differentiation, immune system regulation factors, and DNA repair are candidates to undergo rejection of self target tissues (Figure 3). For example, the chicken showing kDNA mutation with rupture of the dystrophin gene (Figure 5), coding a protein that binds the cytoskeleton to the cell membrane, is a candidate to develop autoimmune inflammatory cardiomyopathy and failure.
These genomic modifications are not seen in chicks hatched from Lb infected eggs. There are differences between T. cruzi and Lb kDNA minicircles; The T. cruzi kDNA minicircle average 1.4 kb structure with four variable region (VR) interspersed by conserved regions (CR) each presenting CSB1, CSB2, and CSB3 regions, in which CA-rich bent DNA are considered specific sites for initiation of replication, transcription, recombination, and for lateral DNA transfer 3, 4. Contrastingly, the Lb kDNA minicircle (average size 820 bp) contains single CR followed by VR. CR has conserved CSB1 (GGGCGT) and CSB2 (CCCCGTTC) blocks, which are different from those in the T. cruzi minicircles 15, 16, and 17. Considering that Lb CSB3 (GGGGTTGGTGTA) shows 12 nts homology to the T. cruzi it is conceivable that either the Lb kDNA minicircle integrates in a much low frequency, which may not be visible by the techniques used, or that it may, possibly, not integrate in the chicken genome at all.
| Primer | Target DNA | Sequence | Tm* |
| S 34 | T. cruzi kDNA | 5' ACA CCA ACC CCA ATC GAA CC 3' | 57.9 |
| S 67 | T. cruzi kDNA | 5' GGT TTT GGG AGG GG(G/C) (G/C)(T/G)T C 3' | 60.1 |
| S 35 | T. cruzi kDNA | 5' ATA ATG TAC GGG (T/G)GA GAT GC 3' | 59.4 |
| S 36 | T. cruzi kDNA | 5' GGT TCG ATT GGG GTT GGT G 3' | 57,9 |
| Lb3 | Lb kDNA | 5' GGG GTT GGT GTA ATA TAG TGG G 3' | 55.9 |
| Lb5 | Lb kDNA | 5' CTA ATT GTG CAC GGG GAG G 3' | 61.4 |
| Gg1 | Gallus gallus | 5' AGC TGA TCC TAA AGG CAG AGC 3' | 60.1 |
| Gg2 | G. gallus | 5' CTG AGC CTC TGC TTT GAA A 3' | 56.8 |
| Gg3 | G. gallus | 5' TTT CAA AGC AGA GGC TCG G 3' | 60.1 |
| Gg4 | G. gallus | 3' GCT CTG CCT TTA GGA TCA GCT 5' | 64.2 |
| Gg5 | G. gallus | 3' AGC AAC TCA GCG TCC ACC TT 5' | 62.3 |
| Gg6 | G. gallus | 3' CTG TTA GCA TGA GGC TTC ACA A 5' | 60.4 |
Table 1. Primes Used in the PCR Amplifications. * Tm = average annealing temperature °C.
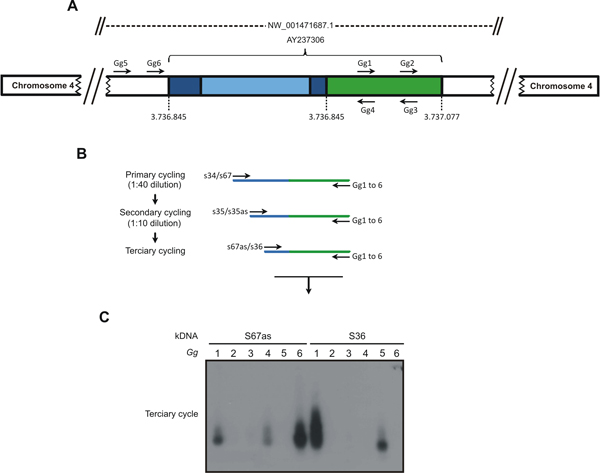
Figure 1. The tpTAIL-PCR strategy used to detect Trypanosoma cruzi kDNA integration into the Gallus gallus genome. A) A chimeric sequence with a fragment of kDNA
minicircle conserved (dark blue) and variable (light blue) regions integrated in the locus
NW_001471687.1 at chromosome 4 (AY237306) of the chicken 10 genome (green)
was used to obtain the host specific primer sets (Gg1 to Gg6). B) The tpTAIL-PCR
amplifications were initiated (primary cycle) by annealing of the minicircle-specific S34 or S67 primers in combination with the chicken-specific Gg1 to Gg6 primers. Diluted products provided template for the secondary cycle with the S35 (sense/antisense) primers and the combinations of Gg primers. In the tertiary cycle a dilution of the secondary products was subjected to amplification with kDNA S36 or S67 antisense primers in combination with the Gg primers. C) These amplification products were separated in 1% agarose gels and transferred to nylon membrane, hybridized with the specific kDNA probe. Samples showing positive signal were used for cloning to determine the point of integration. The combinations of kDNA and
targeted Gg1 to Gg6 are shown on top of the gel. The sequential PCR reactions
amplified target kDNA-host DNA sequences with kDNA minicircles (blue) and the
avian sequence (green). (Reprinted from PLoS Neglected Tropical Diseases 3). Please click here to see a larger version of this figure.

Figure 2. Clinical manifestations of impaired heart function in a 9-month-old chicken genetically modified by the integration of the mitochondrial kDNA minicircle from T. cruzi. The poor blood oxygenation of the mitochondrial kDNA mutated chicken showing a purple comb contrasts with the bright red comb of the control 9-month-old chicken free from heart damage. (Modified from PLoS Neglected Tropical Diseases 3).
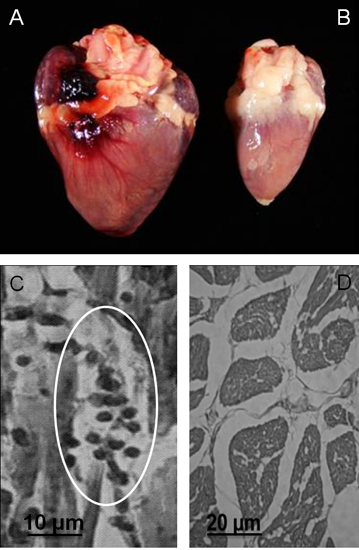
Figure 3. Gross and microscopic pathology in Gallus gallus with kDNA mutations. A) Cardiomegaly in a 9-month-old hen that died of heart failure. B) Control heart from a noninfected 9-month-old hen. C) Rejection of target heart cells by cytotoxic lymphocytes: A minimal rejection unit with lyses of the target cells by immune lymphocytes is depicted (circle). D) Control heart histology (Modified from PLoS Neglected Tropical Diseases 3 ).
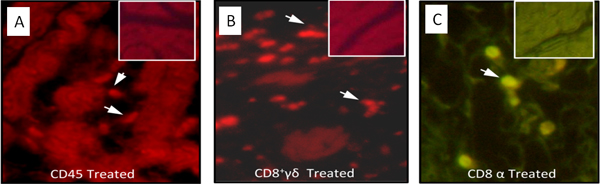
Figure 4. Immunocytochemical analyses of the immune system cells infiltrating the heart of kDNA-mutated chicken shown in Figure 3. A) CD45+ lymphocytes identified (arrows) in heart lesions by a phycoerythrin-labeled specific monoclonal antibody. B) CD8+γδ immune lymphocytes (arrows) involved in severe destruction of the heart. C) Abundant CD8α+ T cells present in severe lesions with heart cell lyses. The inserts show the absence of immune system cells in the control uninfected chicken heart (Modified from PLoS Neglected Tropical Diseases 3 ).

Figure 5. Chagas-like dilated inflammatory cardiomyopathy in a F2 progeny with kDNA integration in the dystrophin gene. A) Dilated heart in a 10-month-old chicken occupying most of the thoracic cavity (heart weight = 16 g). B) Dark round mononuclear cells infiltrates and destroys the myocardium of the kDNA-mutated hen. C) Normal heart size (weight 7 g) of a 10-month-old control chicken. D) Normal histology of a control chicken heart. (Modified from PLoS Neglected Tropical Diseases 3).
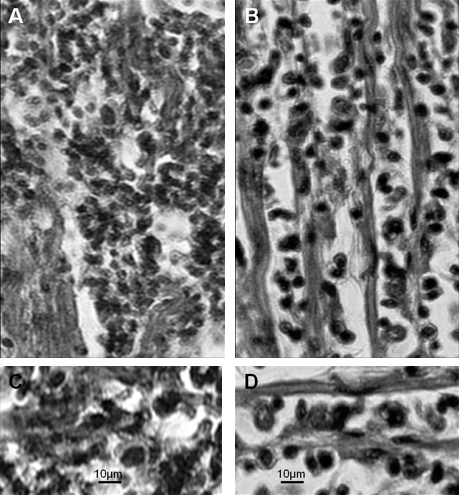
Figure 6. Comparative pathology in kDNA-mutated chicken and in human Chagas disease. A) Severe myocarditis and target heart cell lyses in the kDNA-mutated chicken. B) Severe myocarditis and target cell lyses by immune lymphocytes in a case of Chagas heart disease. C) Rejection of heart cells by immune lymphocytes in the kDNA-mutated chicken. D) Rejection of heart cells by immune lymphocytes in human Chagas disease. Stained by Hematoxilin and Eosin. (Modified from Memórias do Instituto Oswaldo Cruz, Rio de Janeiro 14).
Discussion
In contrast to mammals susceptible to life-long T. cruzi infections, chickens are refractory to T. cruzi infection. The major advantage of the chicken model system is the elimination of the infection early in the development of the embryonic immune system. Thus, the only parasite DNA remaining within the chicken body is integrated at several loci.
Use of the optimal quantity of virulent T. cruzi trypomastigotes to inoculate fertile egg is the critical step towards obtaining integration of the kDNA minicircles into the chicken embryo genome. The rate of live chicks hatching from eggs inoculated with 100 trypomastigotes is four-fold higher than that obtained with 500 parasites. Care should be taken to inoculate the parasites suspension in 10 μL of culture medium into the egg air chamber. There should be no leakage of egg white. Under optimal conditions, the intracellular parasitic infection takes place within a few hours after incubation and parasite multiplication inside the host cells proceeds for one week; thereafter the infection is eliminated by the innate immunity. The kDNA integration requires a living infection, and the inoculation of naked minicircles into early embryo chicken eggs does not result in integration. The kDNA-positive embryos and controls should be housed under controlled conditions at 37.5 °C and 65% humidity. The chicks are kept in cages for two weeks at 33 °C room temperature. Thereafter the chickens are kept in cages on suspended racks separated by 1.5 meter width aisles in a room at 22 °C with filtered air and positive pressure under constant exhaustion to secure animal welfare conditions. The adults are fed chicken-chow and drink potable running water to achieve full growth and maturity, laying eggs at five months of age. Maintenance of hygienic procedures are essential for reproducibility of results when working with T. cruzi inoculated into fertile chicken eggs.
In the chicken model system the T. cruzi infections are eradicated after development of the immune system in the early period of embryo growth. Additionally to being infection-free, the chicks that hatch from T. cruzi inoculated eggs, in lack of specific antibodies, are tolerant to the parasite antigens. The rejection of the target heart cells by cytotoxic lymphocytes (minimal rejection unit, Figure 3) is seen in the kDNA-mutated chickens showing genotype modifications and breakdown of immunological surveillance 3. The genotypically modified T-cells present accelerated rejection of self tissues in the body. The main lesion site is the heart, which is a hallmark of Chagas disease. The passage from a physiologic (surveillance) to a physiopathologic state is seen in the kDNA-mutated chicken showing clonally proliferation of cytotoxic lymphocytes 3.
This transkingdom model system shows a parasite-induced, genetically-driven autoimmune disease (Figure 6), stemming from genome modifications by the T. cruzi kDNA minicircle integrations. These modifications are not seen in chicks hatched from Lb-inoculated eggs.
This phenomenon suggests that experimental treatment of the inflammatory autoimmune cardiomyopathy in kDNA-mutated chickens may require drug suppression of bone marrow progenitor of specific T-cell phenotype infiltrating the myocardium, and transplantation of histocompatible healthy bone marrow to prevent the rejection of self-tissue.
Disclosures
No conflicts of interest declared.
Acknowledgements
We are indebted to Nancy R. Sturm, Department of Immunology, Microbiology and Molecular Biology, David Geffen School of Medicine, University of California at Los Angeles, for critical reading the manuscript. The National Council of Research-CNPq, and the Foundation for Research Development-FAPDF, Brazil, supported the study. We thank the technical aid of Alessandro O. Souza, Maria C. Guimaro, Ciro Cordeiro, Ana de Cassia Rosa, Roseneide Alves, and Rafael Andrade, from the University of Brasilia, Brazil.
Materials
| Name | Company | Catalog Number | Comments |
| Name of the reagent | Company | Catalogue number | Comments |
| Taq DNA Polymerase Recombinant | Invitrogen | 11615-010 | |
| Platinum Taq DNA Polymerase | Invitrogen | 10966-030 | |
| Random Primers DNA Labeling System | Invitrogen | 18187-013 | |
| EcoRI | Invitrogen | 15202-021 | |
| MboI | Invitrogen | 15248-016 | |
| dNTP Set, 100mM Solutions | GE Healthcare | 28-4065-51 | |
| Amersham Hybond – N+ -– Cat n. | GE Healthcare | RPN303B | |
| PlasmidPrep Mini Spin kit | GE Healthcare | 28-9042-70 | |
| NsiI | SIGMA -ALDRICH | R5884 1KU | |
| DNA, Sodium Salt Fish Sperm | AMRESCO | 0644-10G | |
| Mouse anti-chicken Bu-1b | SouthernBiotech | 8370-02 | |
| Mouse anti-chicken CD45 | SouthernBiotech | 8270-08 | |
| Mouse anti-chicken TCRγδ | SouthernBiotech | 8230-08 | |
| Mouse anti-chicken CD8α | SouthernBiotech | 9220-02 | |
| Mouse anti-chicken monocyte/macrophage | SouthernBiotech | 8420-02 | |
| MyCycle Termocycler | Bio-Rad Laboratories | 580BR 5501 |
References
- Teixeira, A. R. Pathogenesis of chagas' disease: parasite persistence and autoimmunity. CMR. 24, 592-630 (2011).
- Teixeira, A. R. Chagas disease. Postg. Med. J. 82, 788-798 (2006).
- Teixeira, A. R. Trypanosoma cruzi in the chicken model: Chagas-like heart disease in the absence of parasitism. PLoS Negl. Trop. Dis. 5, e1000 (2011).
- Hecht, M. M. Inheritance of DNA transferred from American trypanosomes to human hosts. PLoS One. 5, e9181 (2010).
- Xing, Z. Roles of the ERK MAPK in the regulation of proinflammatory and apoptotic responses in chicken macrophages infected with H9N2 avian influenza virus. J. Gen. Virol. 91, 343-351 (2010).
- Kim, H. B. NIK and IKKbeta interdependence in NF-kappaB signalling--flux analysis of regulation through metabolites. Biosystems. 99, 140-149 (2010).
- Karakhanova, S. ERK/p38 MAP-kinases and PI3K are involved in the differential regulation of B7-H1 expression in DC subsets. Eur. J. Immunol. 40, 254-266 (2010).
- Finsterer, J. The heart in human dystrophinopathies. Cardiology. 99, 1-19 (2003).
- Nitz, N. Heritable integration of kDNA minicircle sequences from Trypanosoma cruzi into the avian genome: insights into human Chagas disease. Cell. 118, 175-186 (2004).
- Simpson, L. Kinetoplast DNA in trypanosomid flagellates. Int. Rev. Cytol. 99, 1-19 (1986).
- Bonney, K. M. Heat-killed Trypanosoma cruzi induces acute cardiac damage and polyantigenic autoimmunity. PLoS One. 6, e14571 (2011).
- Moser, D. R. Detection of Trypanosoma cruzi by DNA amplification using the polymerase chain reaction. J. Clin. Microbiol. 27, 1477-1482 (1989).
- Sturm, N. R. Sensitive detection and schizodeme classification of Trypanosoma cruzi cells by amplification of kinetoplast minicircle DNA sequences: use in diagnosis of Chagas' disease. Mol. Biochem. Parasitol. 33, 205-214 (1989).
- Teixeira, A. R. Evolution and pathology in chagas disease--a review. Mem. Inst. Oswaldo Cruz. 101, 463-491 (2006).
- Yurchenko, V. Y. Structure of Leishmania minicircle kinetoplast DNA classes. J. Clin. Microbiol. 37, 1656-1657 (1999).
- Simpson, L. The genomic organization of guide RNA genes in kinetoplastid protozoa: several conundrums and their solutions. Mol. Biochem. Parasitol. 86, 133-141 (1997).
- Thomas, S. A non-universal transcription factor? The Leishmania tarentolae TATA box-binding protein LtTBP associates with a subset of promoters. Int J. Parasitol. 36, 1217-1226 (2006).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved