A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Attaching Biological Probes to Silica Optical Biosensors Using Silane Coupling Agents
In This Article
Summary
Biosensors interface with complex, biological environments and perform targeted detection by combining highly sensitive sensors with highly specific probes attached to the sensor via surface modification. Here, we demonstrate the surface functionalization of silica optical sensors with biotin using silane coupling agents to bridge the sensor and the biological environment.
Abstract
In order to interface with biological environments, biosensor platforms, such as the popular Biacore system (based on the Surface Plasmon Resonance (SPR) technique), make use of various surface modification techniques, that can, for example, prevent surface fouling, tune the hydrophobicity / hydrophilicity of the surface, adapt to a variety of electronic environments, and most frequently, induce specificity towards a target of interest.1-5 These techniques extend the functionality of otherwise highly sensitive biosensors to real-world applications in complex environments, such as blood, urine, and wastewater analysis.2,6-7 While commercial biosensing platforms, such as Biacore, have well-understood, standard techniques for performing such surface modifications, these techniques have not been translated in a standardized fashion to other label-free biosensing platforms, such as Whispering Gallery Mode (WGM) optical resonators.8-9
WGM optical resonators represent a promising technology for performing label-free detection of a wide variety of species at ultra-low concentrations.6,10-12 The high sensitivity of these platforms is a result of their unique geometric optics: WGM optical resonators confine circulating light at specific, integral resonance frequencies.13 Like the SPR platforms, the optical field is not totally confined to the sensor device, but evanesces; this "evanescent tail" can then interact with species in the surrounding environment. This interaction causes the effective refractive index of the optical field to change, resulting in a slight, but detectable, shift in the resonance frequency of the device. Because the optical field circulates, it can interact many times with the environment, resulting in an inherent amplification of the signal, and very high sensitivities to minor changes in the environment.2,14-15
To perform targeted detection in complex environments, these platforms must be paired with a probe molecule (usually one half of a binding pair, e.g. antibodies / antigens) through surface modification.2 Although WGM optical resonators can be fabricated in several geometries from a variety of material systems, the silica microsphere is the most common. These microspheres are generally fabricated on the end of an optical fiber, which provides a "stem" by which the microspheres can be handled during functionalization and detection experiments. Silica surface chemistries may be applied to attach probe molecules to their surfaces; however, traditional techniques generated for planar substrates are often not adequate for these three-dimensional structures, as any changes to the surface of the microspheres (dust, contamination, surface defects, and uneven coatings) can have severe, negative consequences on their detection capabilities. Here, we demonstrate a facile approach for the surface functionalization of silica microsphere WGM optical resonators using silane coupling agents to bridge the inorganic surface and the biological environment, by attaching biotin to the silica surface.8,16 Although we use silica microsphere WGM resonators as the sensor system in this report, the protocols are general and can be used to functionalize the surface of any silica device with biotin.
Protocol
1. Background
The biotin is attached to the surface of these devices through a simple, three-step process (Figure 1). First, we clean the surface and populate it with hydroxyl groups by exposing the devices to either oxygen plasma or piranha solution. Second, we use vapor deposition to attach the silane coupling agent terminated with a primary amine to the hydroxyl groups through hydrolysis and condensation reaction. Third, we attach biotin to the surface via N-hydroxysuccinimide (NHS) ester chemistry. We direct the interested reader to our previous work for more information about the development of these techniques, as well as an explanation of our motivation for choosing these techniques.8
The success of these reactions can be evaluated through optical and fluorescence microscopy after the addition of the primary amine, as well as after the addition of the biotin. While optical microscopy can be used to determine if the surface functionalization protocols resulted in damage to the microsphere surface, or even contamination, fluorescence microscopy is used to verify the quality and uniformity of surface coverage of the biotin molecule, as well as the capability of the biotin on the surface to bind with (strept)avidin. To evaluate the coverage of amines on the surface, we used fluorescein isothiocyanate (FITC) fluorescent dye, which reacts with primary amines to form stable, covalent thiourea linkage to the microsphere. Texas Red fluorescent dye that has been conjugated to avidin is used to label the biotin groups on the surface through the biotin-avidin interaction. In both cases, dyes were chosen that can interact (through either receptor-ligand interactions or covalent bonding) with the functional groups on the surface.
Here, the silane coupling agent, which has three leaving groups and one functional group, provides the bridge between the inorganic surface and organic probe molecule. The primary amine functionality reacts quantitatively with NHS esters to form stable amide bonds. In this case, we use a biotin probe molecule whose valeric side chain has been modified with an NHS ester group. Realistically, any probe molecule to which an NHS ester group can be added to the surface using the following protocols. Additionally, these protocols are general, and can be used to functionalize any silica device surface with biotin.
The primary challenge with these protocols is not actually in the chemistry itself, but rather in the handling of the silica microspheres. Please note that, throughout the protocols, the microspheres should be handled by grapsing their stems lightly with sharp-tipped tweezers. This keeps the tweezers well away from the microspheres themselves, and allows for easy transport between steps. Many steps in the protocol below are specifically designed to address this factor.
2. Microsphere Fabrication
- Build the storage housing for the microspheres.
- Using an exacto knife, cut a ¼ in thick piece of cardboard into a 1 in x 1 in square; tape this onto a regular-sized glass slide, and attach a 1 in section of scotch tape, with its ends meeting to form a roll, to the cardboard.
- This creates a platform on which the microsphere can be elevated and isolated from the surrounding environment, and prevents damage to their surface.
- Using scissors, cut a 3 inch section of optical fiber from a spool of optical fiber.
- Using a No-Nik fiber-stripper, strip the protective polymeric coating from the last 0.5 inches of the end of the cut piece of fiber, leaving just the silica core. Clean the surface of any remaining polymer with a Kimwipe dampened with methanol by gently wiping the fiber with the Kimwipe.
- Using a bare fiber cleaver, trim the stripped end so that only about 1 millimeter of stripped fiber remains on the end of the fiber.
- Place the stripped end of the optical fiber into the path of a CO2 laser, taking care to vertically align the fiber such that its stripped end is facing down.
- Turn on the CO2 laser, and direct it to the surface of the stripped end of the optical fiber. The laser will melt the stripped end of the fiber into a sphere using approximately 3.5% power in 2 seconds.
- Using tweezers, carefully grasp the stem (the non-stripped portion of the optical fiber) of the microsphere. Attach the stem to the tape roll on the microsphere housing. The glass slide may be itself stored in a petri dish. This allows for safe storage of the microsphere.
3. Populating the Surface with Hydroxyl Groups
- Using piranha solution:
- In a 60 mL polypropylene vial with a hinged cap, prepare a piranha solution (70:30 by volume fuming H2SO4:H2O2 (30 wt%)) by adding 5 ml of hydrogen peroxide to the vial, followed by 11.6 mL of fuming sulfuric acid. CAUTION: wear acid-resistant gloves.
- Transfer the glass slide, holding at least 1 microsphere, to the vial. The microsphere should be in contact with the liquid, but the liquid should not touch the cardboard. Adjust the volume of the solution if needed.
- Gently remove the glass slide from the vial, and insert it into another plastic vial containing DDI H2O. Let the samples sit in the solution for 5 minutes.
- Gently remove the glass slide from the vial, and place it an oven at 80 °C for 10 minutes to dry the surface.
- Using oxygen plasma treatment:
- Transfer the glass slide containing at least one microsphere to the oxygen plasma chamber.
- Set the oxygen pressure to 200 mTorr, and the power to 120 W. Expose the sample to the oxygen plasma for 2 minutes.
- Remove the glass slide from the plasma chamber.
4. Attaching Silane Coupling Agents to the Surface
- Place the glass slide, containing at least one microsphere, into a vacuum desiccator in a fume hood.
- Also in the fume hood, open the silane coupling agent bottle (in this case, aminopropyltrimethoxysilane (APTMS) was used), and place the opened bottle in the desiccator.
- Replace the lid on the vacuum desiccator, and attach the outlet port to an aspirator or house vacuum line.
- Turn on the house vacuum or the water line, and evacuate the vacuum desiccator. Once a vacuum seal is formed between the lid and the base, begin timing the reaction. This will deposit the silane coupling agent onto the surface as a thin film.
- After 15 minutes, turn off the vacuum, and slowly open the port to let air into the desiccator. For this coupling agent, 15 minutes is sufficient to form a uniform monolayer on the surface. For other coupling agents, the time may need to be adjusted.
5. Attaching Biotin to the Surface
- Approximately one hour before attaching the biotin, prepare a 10 mM solution of N-hydroxylsuccinimide biotin (NHS-biotin) in anhydrous dimethylsulfoxide (DMSO) in a 60 mL polypropylene vial with a hinged cap.
- If the NHS-biotin has been stored cold, allow it to equilibrate to room temperature before massing it out.
- Sonicate the solution for 1 hour to fully dissolve the NHS-biotin powder in the solvent.
- Transfer the glass slide containing the microsphere into another plastic vial, with the microspheres at the bottom, and stems at the top of the vial.
- Transfer, using a plastic pipet, an appropriate volume of the NHS-biotin solution down the side of the vial, behind the glass slide (so the solution does not touch the microsphere as it is being added to the vial). Add enough solution to cover the microsphere surface.
- Place the vials now containing the microspheres and NHS-biotin in DMSO solution onto a rocking incubator (tilt tray) for 30 minutes at room temperature, with the speed and angle of tilt at 5 rpm and 5 degrees, respectively.
- Gently remove the glass slide from the vial, and gently slide it into another vial filled with DDI H2O. Place the vial on the tilt tray for another 10 minutes at the same speed and tilt angle as before. Repeat this step twice with fresh water each time. This helps remove excess DMSO from the surface, and removes any physically-adsorbed biotin that did not actually graft to the surface.
- Remove the glass slide from the vial, and place it in an oven at 80 °C for 10 minutes, or until all water droplets are removed from the surface.
6. Fluorescent labeling of amine-terminated silica
- To label the amine groups present after treatment with APTMS, prepare the FITC solution in a darkened room by dissolving 1 mg FITC in 1 mL anhydrous DMSO.
- Dilute the solution in 6.1 by adding 50 μL of the FITC solution to 1 mL of 0.1 M sodium bicarbonate buffer.
- Place the solution in a 60 mL polypropylene vial with a hinged cap, and gently slide the amine-terminated microspheres, housed again on the glass slide, into the vial.
- Place the vial in an ice bath, and let the sample react with the FITC solution for a minimum of 4 hours in the dark.
- Remove excess fluorophore through two, 10 minute rinses of the microspheres in sodium carbonate buffer. As before, fill a 60 mL polypropylene vial with a hinged cap with the buffer, and gently slide the glass slide into the solution, taking care that the solution only covers the microsphere, and not the cardboard housing. Cover the vial with aluminum foil, and place the vial on a tilt tray set at 5 degrees and 5 rpm, as before.
- Gently remove the glass slide from the vial, and gently slide it into another vial filled with DDI H2O and covered with aluminum foil. Place the vial on the tilt tray for another 10 minutes at the same speed and tilt angle as before. Repeat this step twice with fresh water each time. This helps remove excess dye from the surface.
- Gently remove the glass slide from the vial, and dry in an oven set at 80 C for 10 minutes before imaging.
7. Fluorescent labeling of biotin-terminated silica
- Prepare a 10 μg/mL solution of Texas-Red avidin in phosphate buffered saline.
- Add the solution to a 60 mL polypropylene vial with a hinged cap, and gently slide the glass slide containing a biotin-terminated microsphere into the solution, so that the microsphere is just covered by the solution.
- React for 30 minutes at room temperature in the dark.
- Remove excess fluorophore through two, 10 minute rinses of the microspheres in PBS buffer.
- As before, fill a 60 mL polypropylene vial with a hinged cap with the buffer, and gently slide the glass slide into the solution, taking care that the solution only covers the microsphere, and not the cardboard housing.
- Cover the vial with aluminum foil, and place the vial on a tilt tray set at 5 degrees and 5 rpm, as before.
- Gently remove the glass slide from the vial, and gently slide it into another vial filled with DDI H2O and covered with aluminum foil. Place the vial on the tilt tray for another 10 minutes at the same speed and tilt angle as before. Repeat this step twice with fresh water each time. This helps remove excess dye from the surface.
- Gently remove the glass slide from the vial, and dry in an oven set at 80 °C for 10 minutes before imaging.
8. Representative Results
Correctly functionalized microspheres can be identified through optical and fluorescence microscopy. If the surface functionalization is done correctly, it should result in a uniformly dense coverage of biotin molecules on the surface, and the surface should remain defect- and contaminant-free after functionalization in order to maintain their high sensitivities during detection experiments. Optical microscopy can be used to probe the latter, while fluorescent microscopy can probe the quality and uniformity of surface coverage. In Figure 2, we show examples of correctly functionalized microspheres. These images show that there is no surface damage or contamination due to functionalization (Fig. 2a), and that the microspheres show a uniform, consistent coverage of either amine groups (Fig. 2b) or biotin groups (Fig. 2c) on the surface.
If the microspheres have not been functionalized correctly, the optical microsphere images will exhibit surface contamination, clumping or non-uniform coverage, and obvious defects in the surface, like surface cracks (Figure 3). Here, we see a common example of surface contamination, resulting from clumping of reagents on the surface.
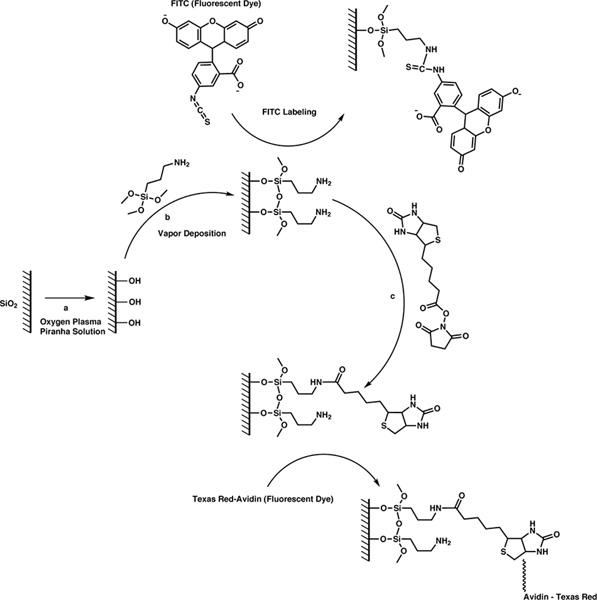
Figure 1. 3-step reaction scheme for attaching probe molecules to the surface of silica microspheres. Click here to view larger figure.
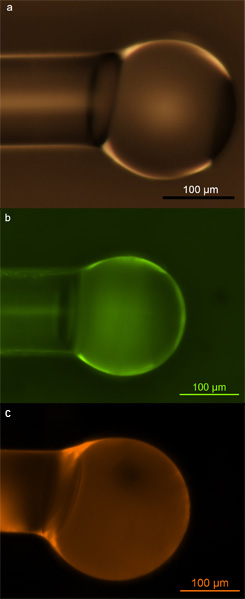
Figure 2. Silica microspheres. a) Optical micrograph of silica microsphere populated with hydroxyl groups via exposure to oxygen plasma; b) Fluorescent micrograph of silica microsphere populated with primary amines and labeled with FITC dye; c) Fluorescent micrograph of silica microsphere populated with biotin and labeled with Texas Red-avidin conjugate. Reprinted with permission from Soteropulos, C. E., Hunt, H. K. & Armani, A. M. Determination of binding kinetics using whispering gallery mode microcavities. Appl. Phys. Lett. 99, 103703-103703 (2011). Copyright 2011, American Institute of Physics.17

Figure 3. Optical micrograph of improperly functionalized sphere. Here, you can see dust on the top right surface, as well as contamination extending off the surface. Additionally, the right side of the optical microsphere shows a small divot on the surface.
Discussion
As described in the protocols, we created a housing platform by which to transport the silica microspheres by their stems throughout the functionalization process. This housing platform was created as a solution to the surface contamination and damage that resulted from the microsphere coming into contact with the walls of the various containers used throughout the functionalization process. We realized the main difficulty arose from constantly attaching and detaching individual microspheres to different containers durin...
Disclosures
No conflicts of interest declared.
Acknowledgements
The authors gratefully acknowledge Prof. Andrea Armani at the University of Southern California for support during the time this protocol was developed. Funding for the initial development of this work was provided by the National Science Foundation [085281 and 1028440] and the National Institute of Health through NIH Director's New Innovator Award Program [1DP2OD007391-01]. Additional information is available at http://web.missouri.edu/~hunthk/.
References
- Datar, R. Cantilever Sensors: Nanomechanical Tools for Diagnostics. MRS Bull. 34, 449-454 (2009).
- Hunt, H. K., Armani, A. M. Label-free biological and chemical sensors. Nanoscale. 2, 1544-1559 (2010).
- Sundberg, F., Karlsson, R. Rapid detection and characterization of immune responses using label-free biacore immunoassays. Immunology. 120, 46-47 (2007).
- Hermanson, G. T. . Bioconjugate Techniques. , (2008).
- Bernards, M. T., Cheng, G., Zhang, Z., Chen, S. F., Jiang, S. Y. Nonfouling polymer brushes via surface-initiated, two-component atom transfer radical polymerization. Macromolecules. 41, 4216-4219 (2008).
- Fan, X. D. Sensitive optical biosensors for unlabeled targets: A review. Anal. Chim. Acta. 620, 8-26 (2008).
- Qavi, A. J., Washburn, A. L., Byeon, J. Y., Bailey, R. C. Label-free technologies for quantitative multiparameter biological analysis. Analytical and Bioanalytical Chemistry. 394, 121-135 (2009).
- Hunt, H. K., Soteropulos, C., Armani, A. M. Bioconjugation Strategies for Microtoroidal Optical Resonators. Sensors. 10, 9317-9336 (2010).
- Kalia, J., Raines, R. T. Advances in Bioconjugation. Curr. Org. Chem. 14, 138-147 (2010).
- Matsko, A. B., Savchenkov, A. A., Strekalov, D., Ilchenko, V. S., Maleki, L. Review of Applications of Whispering-Gallery Mode Resonators in Photonics and Nonlinear Optics. IPN Progress Report. , 42-162 (2005).
- Armani, A. M., Kulkarni, R. P., Fraser, S. E., Flagan, R. C., Vahala, K. J. Label-free, single-molecule detection with optical microcavities. Science. 317, 783-787 (2007).
- Zhu, J. On-chip single nanoparticle detection and sizing by mode splitting in an ultrahigh-Q microresonator. Nat. Photon. 4, 122-122 (2010).
- Armani, D. K., Kippenberg, T. J., Spillane, S. M., Vahala, K. J. Ultra-high-Q toroid microcavity on a chip. Nature. 421, 925-928 (2003).
- Vollmer, F., Arnold, S. Whispering-gallery-mode biosensing: label-free detection down to single molecules. Nat. Methods. 5, 591-596 (2008).
- Vollmer, F., Arnold, S., Keng, D. Single virus detection from the reactive shift of a whispering gallery mode. Proc. Natl. Acad. Sci. U.S.A. 105, 20701-20704 (2008).
- Hunt, H. K., Armani, A. M. Recycling microcavity optical biosensors. Opt. Lett. 36, 1092-1094 (2011).
- Soteropulos, C. E., Hunt, H. K., Armani, A. M. Determination of binding kinetics using whispering gallery mode microcavities. Appl. Phys. Lett. 99, 103703-103703 (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved