A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Clinical Testing and Spinal Cord Removal in a Mouse Model for Amyotrophic Lateral Sclerosis (ALS)
In This Article
Summary
A mouse model for amyotrophic lateral sclerosis (ALS) is examined clinically and behaviorally. As a prerequisite for an accompanying immunohistological analysis the preparation of the spinal cord is depicted in detail.
Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disorder resulting in progressive degeneration of motoneurons. Peak of onset is around 60 years for the sporadic disease and around 50 years for the familial disease. Due to its progressive course, 50% of the patients die within 30 months of symptom onset. In order to evaluate novel treatment options for this disease, genetic mouse models of ALS have been generated based on human familial mutations in the SOD gene, such as the SOD1 (G93A) mutation. Most important aspects that have to be evaluated in the model are overall survival, clinical course and motor function. Here, we demonstrate the clinical evaluation, show the conduction of two behavioural motor tests and provide quantitative scoring systems for all parameters. Because an in depth analysis of the ALS mouse model usually requires an immunohistochemical examination of the spinal cord, we demonstrate its preparation in detail applying the dorsal laminectomy method. Exemplary histological findings are demonstrated. The comprehensive application of the depicted examination methods in studies on the mouse model of ALS will enable the researcher to reliably test future therapeutic options which can provide a basis for later human clinical trials.
Protocol
Animals were purchased from Jackson Laboratory (# 002726) 1. They are clinically scored and subjected to a test of motor function (rotarod test) and of muscular strength (hanging wire test). All these tests and the later killing of the animals in order to prepare the spinal cord have been performed in very close accordance to the local guidelines for proper conduct of animal experiments.
1. Clinical Score
Apart from assessment for body weight mice are examined for signs of motor deficit with the following 4 point scoring system 2:
4 points: normal (no sign of motor dysfunction)
3 points: hind limb tremors are evident when suspended by the tail
2 points: gait abnormalities are present
1 point: dragging of at least one hind limb
0 point: symmetrical paralysis, inability to right itself or loss of 20% of maximum body weight; in this case the animals are immediately euthanized and the experiment is terminated
2. Tests of Motor Function and Muscular Strength
Hanging wire
This test is used to assess muscular strength 3, 4. All animals perform this test at least one or two days after the rotarod test. Each mouse is placed on a custom-made wire lid with intervals of 0.8 cm and cautiously turned upside down, 60 cm above a straw covered bottom. After training for three consecutive times of at least 180 s the latency to fall is measured. Each mouse is given up to three attempts to hold on to the inverted lid for a maximum of 180 s and the longest period is recorded.
Rotarod Test
The rotarod apparatus (Ugo Basile, Comerio, Italy) was used to measure motor coordination, balance and motor learning ability 3, 4. A good performance requires a high degree of sensorimotor coordination. The machine should be placed in a calm and non-disturbing environment to avoid distractive stimuli for the tested animal. It consists of a computer-controlled motor-driven rotating spindle and five lanes for five mice. Falls of the mice are detected automatically by pressure on a plastic plate at the bottom. After training for three consecutive times of at least 180 s at a constant speed of 15 r.p.m. the time for which an animal can remain on the rotating rod is measured. Each animal undergoes three trials and the longest latency without falling is recorded. The time of 180 s is chosen as cut-off time because the majority of significant differences in motor coordination are detected in this time frame.
3. Spinal Cord Preparation
- Animals are killed by CO2 insufflation in accordance with the local guidelines and are immediately perfused transcardially with PBS solution followed by a 4% paraformaldehyde solution.
- In order to prepare the spinal cord of the sacrificed mouse, the animal is placed onto an operation table and the four limbs are fixed on the side in order to expose the back side of the mouse.
- A short wash with a 70% ethanol solution cleans the site of dissection and flattens the hair coat.
- Then the skin is incised with a sharp scalpel in the median line. In order to facilitate cutting the skin is stretched to both sides. If leg muscles shall be prepared, their skin has also to be incised.
- After the skin incision is completed, it is pulled aside with a pair of tweezers to expose the underlying superficial fascia of the body.
- The musculature of the neck and the nuchal ligament have to be removed and are carefully prepared. Be careful not to incise to deeply and lesion the spinal cord. The shoulder muscles can also be removed in order to better expose the spinal column.
- Then the paravertebral muscles are removed from the entire spinal column.
- In order to open the spinal column several laminectomies have to be performed. One should start from the upper cranial part at the site of the atlanto-occipital joint.
- It is easiest to remove the fixation of the upper two limbs and overstretch the neck to be better able to perform the laminectomy of the first vertebrae. These are pulled away without touching the exposed cervical spinal cord.
- More vertebrae are removed by first transecting the vertebral arches at both sides with angled scissors and then pulling on the dorsal processes. Remaining lateral parts of the vertebrae should be removed to facilitate later complete removal of the spinal cord.
- An anatomical landmark of the lumbar spinal cord is the intumescence that is also present in the cervical spinal cord.
- Having finished the laminectomy of the entire spinal cord, make sure that you also transect all ventral roots and release the spinal cord from the dura mater of the meninges.
- Then the cervical spinal cord is cut cranially and you start to remove the spinal cord.
- Finally the spinal cord is also cut at the distal cauda-equina to be completely released.
- Ultimately, the spinal cord is placed into a postfixating solution (e.g. 4% paraformaldehyde) overnight and can be further processed. We usually cryosection the spinal cord to prepare it for immunohistological analysis.
4. Representative Results
The technique of spinal cord preparation represents the focus of this video article. It is an essential prerequisite for later tissue sectioning and ultimately for immunohistological analysis of spinal cord sections. As an example of a final result, an immunohistochemical workup of the anterior horn region of the mouse lumbar spinal cord of a wildtype (wt) and of a SOD G93A transgene (tg) mouse is demonstrated. Motor neurons can be identified with a primary anti-ChAT antibody and subsequent fluorescent labeling with a secondary Cy3 antibody. In addition, a nuclear counter-stain with DAPI (4,6-diamidino-2-phenylindole) has been performed (Figure 1).
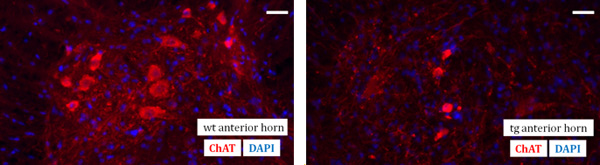
Figure 1. Fluorescent photomicrographs visualizing the immunodetection of motoneurons with anti-ChAT antibody (red) and cellular nuclei counter-stain by DAPI (blue) in the mouse lumbar spinal cord anterior horn of a wildtype (wt) (on the left) and of a SOD G93A transgenic (tg)(on the right) mouse at the age of 130 days. Scale bar: 40 μm.
As the immunohistochemical analysis of the SOD G93A mice is not the primary scope of this article please consult the original publication in which these transgenic mice have been characterized and more recent ones which study therapeutic approaches for further reference 1, 5, 6. If therapeutic effects shall be differentiated on the immunohistological level clearly defined quantitative evaluation algorithms should be applied supported by a stereological software (for example see 7).
Discussion
The SOD1 (G93A) genetic mouse model is a valuable animal model to study the disease course of progressive motoneuron loss comparable to human amyotrophic lateral sclerosis 8. A variety of different treatment paradigms have been evaluated in this model and represent a basis for later testing in human clinical studies 8-10. In order to be able to detect significant differences in an experimental treatment study in these mice, it is of eminent importance to include at least 24 litter-matched gender-bal...
Disclosures
No conflicts of interest declared.
Acknowledgements
LT has received grant support from the Forschungsförderungsprogramm of the University Medicine Göttingen. PL and MB were supported by the DFG Research Center for Molecular Physiology of the Brain (CMPB), Göttingen. The authors thank Dr. Lars Tatenhorst for assistance with videography and Birgit Liebau for help with audio and video editing.
Materials
| Name | Company | Catalog Number | Comments |
| Rota-Rod for Mice | Ugo Basile | # 47600 | |
| Hanging wire device | Custom Made | ||
| Operation Table Operation lamp Protective gloves | |||
| “Iris” Scissors, angled to side | Fine Science Tools | 14063-09 | |
| Cohan-Vannas Spring Scissors, straight | Fine Science Tools | 15000-10 | |
| Micro forceps | Hammacher, Solingen, Germany | HWC 111-10 | |
| Scalpel “präzisa plus” | Dahlhausen, Köln, Germany | 11.000.00.510, FIG 10 |
References
- Gurney, M. E. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 264 (5166), 1772-1775 (1994).
- Weydt, P. Assessing disease onset and progression in the SOD1 mouse model of ALS. Neuroreport. 14 (7), 1051-1054 (2003).
- Crawley, J. N. Behavioral phenotyping strategies for mutant mice. Neuron. 57 (6), 809-818 (2008).
- Miana-Mena, F. J. Optimal methods to characterize the G93A mouse model of ALS. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 6 (1), 55-62 (2005).
- Zhong, Z. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J. Clin. Invest. 119 (11), 3437-3449 (2009).
- Pitzer, C. Granulocyte-colony stimulating factor improves outcome in a mouse model of amyotrophic lateral sclerosis. Brain. 131 (Pt. 12), 3335-3347 (2008).
- Gowing, G. Ablation of proliferating microglia does not affect motor neuron degeneration in amyotrophic lateral sclerosis caused by mutant superoxide dismutase. J. Neurosci. 28 (41), 10234-10244 (2008).
- Scott, S. interpretation of studies in the standard murine model of ALS. Amyotroph Lateral Scler. 9 (1), 4-15 (2008).
- Turner, B. J., Talbot, K. Transgenics, toxicity and therapeutics in rodent models of mutant SOD1-mediated familial ALS. Prog Neurobiol. 85 (1), 94-134 (2008).
- Corse, A. M. Preclinical testing of neuroprotective neurotrophic factors in a model of chronic motor neuron degeneration. Neurobiol Dis. 6 (5), 335-346 (1999).
- Knippenberg, S. Significance of behavioural tests in a transgenic mouse model of amyotrophic lateral sclerosis (ALS). Behav Brain Res. 213 (1), 82-87 (2010).
- Burgess, R. W., Cox, G. A., Seburn, K. L. Neuromuscular disease models and analysis. Methods Mol. Biol. 602, 347-393 (2010).
- Hayworth, C. R., Gonzalez-Lima, F. Pre-symptomatic detection of chronic motor deficits and genotype prediction in congenic B6.SOD1(G93A) ALS mouse model. Neuroscience. 164 (3), 975-985 (2009).
- Ludolph, A. C. Guidelines for preclinical animal research in ALS/MND: A consensus meeting. Amyotroph Lateral Scler. 11 (1-2), 38-45 (2010).
- Boillee, S. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 5778 (3), 1389-1392 (2006).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved