A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Hydrophobic Salt-modified Nafion for Enzyme Immobilization and Stabilization
In This Article
Summary
This article will describe the procedure for synthesizing a hydrophobically modified Nafion enzyme immobilization membrane and how to immobilize proteins and/or enzymes within the membrane and test their specific activity.
Abstract
Over the last decade, there has been a wealth of application for immobilized and stabilized enzymes including biocatalysis, biosensors, and biofuel cells.1-3 In most bioelectrochemical applications, enzymes or organelles are immobilized onto an electrode surface with the use of some type of polymer matrix. This polymer scaffold should keep the enzymes stable and allow for the facile diffusion of molecules and ions in and out of the matrix. Most polymers used for this type of immobilization are based on polyamines or polyalcohols - polymers that mimic the natural environment of the enzymes that they encapsulate and stabilize the enzyme through hydrogen or ionic bonding. Another method for stabilizing enzymes involves the use of micelles, which contain hydrophobic regions that can encapsulate and stabilize enzymes.4,5 In particular, the Minteer group has developed a micellar polymer based on commercially available Nafion.6,7 Nafion itself is a micellar polymer that allows for the channel-assisted diffusion of protons and other small cations, but the micelles and channels are extremely small and the polymer is very acidic due to sulfonic acid side chains, which is unfavorable for enzyme immobilization. However, when Nafion is mixed with an excess of hydrophobic alkyl ammonium salts such as tetrabutylammonium bromide (TBAB), the quaternary ammonium cations replace the protons and become the counter ions to the sulfonate groups on the polymer side chains (Figure 1). This results in larger micelles and channels within the polymer that allow for the diffusion of large substrates and ions that are necessary for enzymatic function such as nicotinamide adenine dinucleotide (NAD). This modified Nafion polymer has been used to immobilize many different types of enzymes as well as mitochondria for use in biosensors and biofuel cells.8-12 This paper describes a novel procedure for making this micellar polymer enzyme immobilization membrane that can stabilize enzymes. The synthesis of the micellar enzyme immobilization membrane, the procedure for immobilizing enzymes within the membrane, and the assays for studying enzymatic specific activity of the immobilized enzyme are detailed below.
Protocol
1. Modification of Nafion with Quaternary Ammonium Salts
- Shake a bottle of 5% w/v Nafion suspension vigorously for approx. 30 seconds to ensure that Nafion is suspended uniformly in solution.
- Pipette out 2 mL of the now re-suspended Nafion into a glass vial (vial volume could contain from 2.5 mL to 10 mL).
- Measure a 3-fold molar excess (relative to the sulfonic acid groups on the Nafion polymer) of the alkyl ammonium bromide salt (appropriate masses are shown in Table 1) and add this to the vial containing the 2 mL of Nafion.
- Vortex the vial at 1500 rpm for 10-15 minutes.
- Pour the viscous solution into a plastic weighing tray that measures approx. 3 x 3 in., and use a pipette to transfer any residual solution from the vial to the weighing tray.
- Allow the solvents to evaporate out of the weigh boat, leaving a yellow/brown, transparent film on the bottom of the weighing tray (Figure 2). The rate of solvent evaporation should be such that it takes more than 6 hours for all solvent to evaporate. If the solvent evaporates too fast, a white, crusty material will form instead of a transparent film indicating that the micellar structure of the polymer has been destroyed, and you must re-start the procedure. If solvent evaporation is too slow, a de-humidifier may be necessary, because too slow of evaporation typically leads to a tacky, orange gel and you must re-start the procedure. Typical temperature ranges for solvent evaporation are 20 - 37 °C. The actual conditions for drying are a function of the relative humidity and temperature in the room, but it is important that drying being slow to maintain micelle structure, but not too slow to allow for gel formation.
- Fill the weigh boat with 18M Ωcm de-ionized water (10-20 mL of water), cover, and allow to soak for 12-24 hours to remove excess alkyl ammonium bromide salts and HBr.
- Pour (or pipette) out the water and rinse 3 times with enough DI water to fill the tray each time. Be careful not to lose any of the polymer film during this step.
- Allow the weighing tray to sit uncovered until the polymer is completely dry At this point, the polymer should be a clear and somewhat brittle plastic film. Again, if the air is very humid, a de-humidifier may be necessary to complete the evaporation in a timely manner.
- Using a spatula, carefully remove the dried film from the weighing tray and transfer it into a clean glass vial.
- Add 2 mL of ethanol and 3 ceramic mixing beads, and vortex for 4 hours or until the polymer film is completely re-suspended.
2. Immobilization of Enzymes into TBAB-Modified Nafion for Activity Assays
- For a dry enzyme, weigh out 1-10 mg of the enzyme into a 1.5 mL microcentrifuge tube, and add 1 mL of 100 mM pH 7 phosphate buffer to create a 1-10 mg/mL enzyme solution. For an enzyme that is in solution, use a bicinchoninic acid (BCA) assay13 to determine the amount of protein, and add an appropriate amount of 100 mM phosphate buffer to bring the protein concentration to 1-10 mg/mL. 1-10 mg/mL typically corresponds to 1-50 nanomoles/mL.
- To 120 μL of the 1 mg/mL enzyme solution, add 60 μL of the alkyl ammonium-modified Nafion solution, and vortex for 10 seconds. (This mixture can be scaled up for large numbers of replicates. Keep the enzyme-to-polymer solution ratio at 2:1.)
- Pipette 60 μL of the enzyme/polymer solution into the bottom of 3 separate 1 cm2 cuvettes, and allow to dry overnight.
3. Assay of Immobilized NAD-Dependent Dehydrogenase Enzyme
- To the cuvette, add 1.3 mL of 50 mM sodium pyrophosphate (pH 8.8), 1.5 mL of 15 mM NAD (freshly prepared), and 0.1 mL water.
- Place the cuvette in a UV/Vis spectrophotometer (i.e. ThermoScientific Evolution 260 Bio and Thermo Spectronic Genesys 20) set to a wavelength of 340 nm.
- Zero the spectrometer, and then add 0.1 mL ethanol. Mix the reagents by gently pipetting the solution up and down 5 times. For a blank, use 0.1 mL of additional water instead of the 0.1 mL of ethanol.
- Record the absorbance at 340 nm at 5 minutes after the reagents were added to the cuvette and 20 minutes after. Plot the two data points to get a slope that can be used for activity calculations.
4. Assay of Immobilized PQQ-Dependent Dehydrogenases
- To the cuvette, add 1.5 mL of sodium phosphate (pH 7.3) and 200 μL of 600 μM PMS.
- Place the cuvette in a UV/Vis spectrophotometer set to a wavelength of 600 nm and then zero the spectrometer.
- Add 100 μL of 700 μL DCIP and 200 μL of the substrate of interest (ethanol, acetaldehyde, glycerol, glucose, or glyceraldehyde), and mix the reagents by gently pipetting the solution up and down 5 times. For a blank, use 200 μL of water instead of the substrate of interest.
- Record the absorbance at 600 nm at 5 minutes after the reagents were added to the cuvette and 20 minutes after.
5. Assay of Immobilized Glucose Oxidase
- To the cuvette, add 2.0 mL of a solution containing 0.2 M p-hydroxybenzoic acid, 0.02 % (w/v) sodium azide, 128 U peroxidase, 0.3 mM 4-aminoantipyrine, 1 M potassium phosphate, and 50 mM glucose. Mix the solution by pipetting up and down 5 times.
- Place the cuvette in a UV/Vis spectrophotometer set to a wavelength of 510 nm.
- Record the absorbance at 510 nm at 5 minutes after the reagents were added to the cuvette and again at 20 minutes after.
6. Representative Results
The micellar structure of the modified Nafion polymer can be disrupted by drying the original salt/polymer co-casted film too fast. Figure 2 shows a salt/polymer mixture that has been dried correctly resulting in a transparent, light brown film. A film that dries too fast can result in opaque, white flakes of polymer due to the fact that the drying process can destroy the micellar structure.
Once the modified Nafion polymer and enzyme have been mixed and co-cast onto the bottom of a cuvette, enzymatic activity assays can be used to assess the stability of the enzyme within the polymer film. Tables 2-4 show assay results of two dehydrogenase enzymes and glucose oxidase immobilized into various modified Nafion films, respectively. Note the higher activity of the enzymes that are immobilized vs. the enzymes in buffer solution, showing that modified Nafion polymers can actually enhance the activity of certain enzymes (called superactivity). Other enzymes have transport limitations that decrease their specific activity when immobilize them in the polymer (i.e. cellulases and amylases, whose substrates are quite large macromolecules).
| Quaternary ammonium salt used | 3 fold excess |
| T3A (tetrapropylammonium bromide) | 32.37 mg/ml |
| TBAB (tetrabutylammonium bromide) | 39.19 mg/ml |
| TPAB (tetrapentylammonium bromide) | 46.01 mg/ml |
| TEHA (triethylhexylammonium bromide) | 32.37 mg/ml |
| TMHA (trimethylhexylammonium bromide) | 27.25 mg/ml |
| TMOA (trimethyloctylammonium bromide) | 30.66 mg/ml |
| TMDA (trimethyldecylammonium bromide) | 34.07 mg/ml |
| TMDDA (trimethyldodecylammonium bromide) | 37.48 mg/ml |
| TMTDA (trimethyltetradecylammonium bromide) | 40.89 mg/ml |
| TMHDA (trimethylhexadecylammonium bromide) | 44.31 mg/ml |
| TMODA (trimethyloctadecylammonium bromide) | 47.71 mg/ml |
Table 1. Amounts of tetra-alkyl ammonium salts to use for Nafion polymer modification.
| Type of Nafion | Enzyme activity (U/g) |
| Buffer (no polymer) | 16.63 ± 8.11 |
| Nafion (un-mod.) | 9.25 ± 2.21 |
| TMTDA | 3.23 ± 2.92 |
| TBAB | 3.93 ± 3.33 |
| TMDDA | 4.19 ± 1.04 |
| TMOA | 3.51 ± 1.11 |
| TMDA | 8.00 ± 4.53 |
| TMHA | 1.68 ± 1.39 |
| TMHDA | 4.83 ± 0.99 |
| TMODA | 10.45 ± 3.20 |
Table 2. NAD-dependent glucose dehydrogenase activity immobilized in selected modified Nafion polymers (note: immobilized activity is a function of initial specific activity of the enzyme).
| Type of Nafion | Enzyme activity (mU/g) |
| Buffer (no polymer) | 7.18 ± 0.51 |
| Nafion (un-mod.) | 70.1± 0.5 |
| TMTDA | 133 ± 6 |
| TBAB | 244 ± 4 |
| TMDDA | 221 ± 6 |
| TMOA | 1.78 ± 0.63 |
| TMDA | 206 ± 5 |
| TEHA | 40.1 ± 50.6 |
| TMHDA | 0 |
| TMODA | 1.45 ± 0.06 |
Table 3. PQQ-dependent glucose dehydrogenase activity immobilized in selected modified Nafion polymers (note: immobilized activity is a function of initial specific activity of the enzyme).
| Type of Nafion | Enzyme activity (U/g) |
| Buffer (no polymer) | 103.61 ± 3.15 |
| Nafion (un-mod.) | 19.93 ± 10.10 |
| TMTDA | 247.25 ± 12.49 |
| TBAB | 152.27 ± 5.29 |
| TMDDA | 262.05 ± 6.26 |
| TMOA | 129.18 ± 2.31 |
| TMDA | 141.23 ± 1.97 |
| TMHA | 131.75 ± 2.89 |
| TMHDA | 132.50 ± 1.18 |
| TMODA | 136.50 ± 0.96 |
Table 4. Representative glucose oxidase specific activity immobilized in selected modified Nafion polymers (note: immobilized activity is a function of initial specific activity of the enzyme).
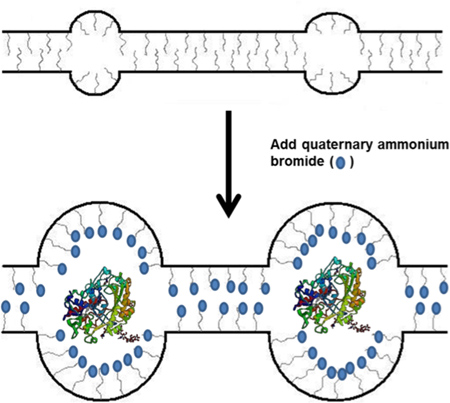
Figure 1. Schematic of TBAB incorporation into Nafion polymer and subsequent use in enzyme immobilization.

Figure 2. Optical photograph of initial co-cast films of Nafion and TBAB. Slow drying yields a transparent, light brown film covering the bottom of the weighing tray.
Discussion
In the described procedure, tetra-alkyl ammonium salts are used to modify commercial Nafion to create micellar polymers that can be used to immobilize and stabilize enzymes. The assays described in the procedure show that the polymer can be used to immobilize a wide variety of enzymes with a high retention of activity. If the enzyme of interest has very low activity or is impure, a higher concentration may be required and should not affect the immobilization process, unless immobilizing enzymes in concentrations ...
Disclosures
No conflicts of interest declared.
Acknowledgements
The authors acknowledge the Office of Naval Research, United Soybean Board, and National Science Foundation for funding.
Materials
| Name | Company | Catalog Number | Comments |
| Nafion | Sigma-Aldrich | 70160 | |
| Tetra alkylammonium bromide salts | Sigma-Aldrich | n/a | |
| Alcohol dehydrogenase | Sigma-Aldrich | A3263 | |
| Nicotinamide adenine dinucleotide (NAD) | Simga-Aldrich | N7004 | |
| Sodium pyrophosphate | Sigma-Aldrich | P8010 | |
| Phenazine methosulfate (PMS) | Sigma-Aldrich | P9625 | |
| 2,6-Dichloroindophenol (DCIP) | Sigma-Aldrich | D1878 | |
| Glucose oxidase | Sigma-Aldrich | G7141 | |
| 4-Hydroxybenzoic acid | Sigma-Aldrich | 240141 | |
| Sodium azide | Sigma-Aldrich | S8032 | |
| Peroxidase | Sigma-Aldrich | P8375 | |
| 4-aminoantipyrine | Sigma-Aldrich | 06800 | |
| UV/Vis Spectrophotometer | Thermo | Evolution 260 Bio or Spectronic Genesys 20 | |
| Vortex Genie | |||
| Analytical balance |
References
- Calabrese-Barton, S., Gallaway, J., Atanassov, P. Enzymatic biofuel cells for implantable and microscale devices Chem. Rev. 104, 4867-4886 (2004).
- Cracknell, J. A., Vincent, K. A., Armstrong, F. A. Enzymes as Working or Inspirational Electrocatalysts for Fuel Cells and Electrolysis. Chem. Rev. 108, 2439-2461 (2008).
- Minteer, S. D., Liaw, B. Y., Cooney, M. J. Enzyme-Based Biofuel Cells. Curr. Opin. Biotechnol. 18, 228-234 (2007).
- Callahan, J. W., Kosicki, G. W. The Effect of Lipid Micelles on Mitochondrial Malate Dehydrogenase. Canadian Journal of Biochemistry. 45, 839-851 (1967).
- Martinek, K. Modeling of the Membrane Environment of Enzymes: Superactivity of Laccase Entrapped into Surfactant Reversed Micelles in Organic Solvents. Biokhimiya. 53, 1013-1016 (1988).
- Moore, C. M., Akers, N. L., Hill, A. D., Johnson, Z. C., Minteer, S. D. Improving the Environment for Immobilized Dehydrogenase Enzymes by Modifying Nafion with Tetraalkylammonium Bromides. Biomacromolecules. 5, 1241-1247 (2004).
- Schrenk, M. J., Villigram, R. E., Torrence, N. J., Brancato, S. J., Minteer, S. D. Effects of Mixture Casting Nafion with Quaternary Ammonium Bromide Salts on the Ion-Exchange Capacity and Mass Transport in the Membranes. J. Membr. Sci. 205, 3-10 (2002).
- Akers, N. L., Moore, C. M., Minteer, S. D. Development of Alcohol/O2 Biofuel Cells Using Salt-Extracted Tetrabutylammonium Bromide/Nafion Membranes to Immobilize Dehydrogenase Enzymes. Electrochim. Acta. 50, 2521-2525 (2005).
- Sokic-Lazic, D., Minteer, S. D. Citric Acid Cycle Biomimic on a Carbon Electrode. Biosens. Bioelectron. 24, 939-944 (2008).
- Arechederra, R. L., Minteer, S. D. Complete Oxidation of Glycerol in an Enzymatic Biofuel Cell. Fuel Cells. 9, 63-69 (2009).
- Germain, M., Arechederra, R. L., Minteer, S. D. Nitroaromatic Actuation of Mitochondrial Bioelectrocatalysis for Self-Powered Explosive Sensors. J. Am. Chem. Soc. 130, 15272-15273 (2008).
- Addo, P. K., Arechederra, R. L., Minteer, S. D. Evaluating Enzyme Cascades for Methanol/Air Biofuel Cells Based On NAD+-Dependent Enzymes. Electroanalysis. 22, 807-812 (2010).
- Smith, P. K., Krohn, R. I., Hermanson, G. T., Mallia, A. K., Gartner, F. H., Provenzano, M. D., Fujimoto, E. K., Goeke, N. M., Olson, B. J., Klenk, D. C. Measurement of protein using bicinchoninic acid. Analytical Biochemistry. 150, 76-85 (1985).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved