A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
High-throughput Synthesis of Carbohydrates and Functionalization of Polyanhydride Nanoparticles
In This Article
Summary
In this article, a high throughput method is presented for the synthesis of oligosaccharides and their attachment to the surface of polyanhydride nanoparticles for further use in targeting specific receptors on antigen presenting cells.
Abstract
Transdisciplinary approaches involving areas such as material design, nanotechnology, chemistry, and immunology have to be utilized to rationally design efficacious vaccines carriers. Nanoparticle-based platforms can prolong the persistence of vaccine antigens, which could improve vaccine immunogenicity1. Several biodegradable polymers have been studied as vaccine delivery vehicles1; in particular, polyanhydride particles have demonstrated the ability to provide sustained release of stable protein antigens and to activate antigen presenting cells and modulate immune responses2-12.
The molecular design of these vaccine carriers needs to integrate the rational selection of polymer properties as well as the incorporation of appropriate targeting agents. High throughput automated fabrication of targeting ligands and functionalized particles is a powerful tool that will enhance the ability to study a wide range of properties and will lead to the design of reproducible vaccine delivery devices.
The addition of targeting ligands capable of being recognized by specific receptors on immune cells has been shown to modulate and tailor immune responses10,11,13 C-type lectin receptors (CLRs) are pattern recognition receptors (PRRs) that recognize carbohydrates present on the surface of pathogens. The stimulation of immune cells via CLRs allows for enhanced internalization of antigen and subsequent presentation for further T cell activation14,15. Therefore, carbohydrate molecules play an important role in the study of immune responses; however, the use of these biomolecules often suffers from the lack of availability of structurally well-defined and pure carbohydrates. An automation platform based on iterative solution-phase reactions can enable rapid and controlled synthesis of these synthetically challenging molecules using significantly lower building block quantities than traditional solid-phase methods16,17.
Herein we report a protocol for the automated solution-phase synthesis of oligosaccharides such as mannose-based targeting ligands with fluorous solid-phase extraction for intermediate purification. After development of automated methods to make the carbohydrate-based targeting agent, we describe methods for their attachment on the surface of polyanhydride nanoparticles employing an automated robotic set up operated by LabVIEW as previously described10. Surface functionalization with carbohydrates has shown efficacy in targeting CLRs10,11 and increasing the throughput of the fabrication method to unearth the complexities associated with a multi-parametric system will be of great value (Figure 1a).
Protocol
1. High-throughput Carbohydrate Synthesis
- Prior to the automated synthesis of dimannoside, a suitably protected sugar donor, typically trichloroacetimidate, and acceptor, mainly an alkenyl fluorous alcohol, are synthesized on the bench-top.
- A program is written for the automated synthesis of dimannoside. A schematic representation of the basic automated procedure is presented in Figure 2. In the program, it is ensured that before the addition of the promoter, the mixture of donor and acceptor is stirred for at least 30 min.
- Solutions of the synthetic donor, acceptor and trimethylsilyltrifluoromethanesulfonate are made in dichloromethane. Toluene and dichloromethane are used most frequently for glycosylation reactions.
- Also, prepare solutions of reagents for the deprotection of temporary protecting groups in 80% methanol and 100% methanol.
- Prior to the start of the program, ensure that the relative humidity in the room is 30% or lower in the automation chamber. High humidity is detrimental for glycosylation reactions.
- Once the program is started, the robotic arm transfers the solutions of donor and acceptor into the reaction vial sequentially. Then the mixture is stirred for 30 min.
- Next the robotic arm transfers 0.2 to 0.3 equivalents of trimethylsilyltrifluoromethanesulfonate into the mixture, typically at room temperature although lower temperatures like -20 °C can be attained. The reaction mixture is stirred for 30 min.
- After 30 min, the reaction is stopped and a small aliquot removed to monitor the reaction progress. If not complete, the reaction can be continued and ultimately the time required can be modified.
- Once the reaction is complete, the reaction mixture is transferred to the fluorous solid phase extraction (FSPE) cartridges containing C8F17-modified silica gel for purification.
- The cartridges are first washed with an 80% methanol-water mixture (8 mL) to get rid of the non-fluorous fraction.
- Then the cartridges are washed with 100% methanol to obtain the desired fluorous-tagged product. If additional purification is desired, the machine can be stopped and the reaction product(s) removed for purification by additional means.
- After the purification cycle, the robotic arm dispenses sodium methoxide into the reaction vial. The reaction is stirred for 2 hr. If not complete, the reaction can again be continued for a longer period and ultimately the programmed time required can be modified.
- After the completion of the reaction, the product is purified by FSPE and then subjected to dissolution in anhydrous toluene followed by evaporation to remove residual water.
- Then the cycle (from step 6 to 13) is repeated till the desired chain length is obtained for the target molecule.
- The protected product obtained from automation is then further purified and fully characterized by techniques such as nuclear magnetic resonance (NMR) spectroscopy. Complete deprotection (removal of all remaining protecting groups) of the final target molecule is then completed outside the automation platform as a rule because it usually involves explosive hydrogen gas and palladium. The final deprotection step was carried out on bench-top outside the automation platform. The first step was ozonolysis of the double bond in fluorous tag followed by oxidation of the produced aldehyde to a carboxylic acid. The product was purified by column chromatography. The final step was deprotection of benzyl ether groups by palladium-catalyzed hydrogenation. The product was passed through celite pad to get rid of palladium to get pure final product.
2. High-throughput Nanoparticle Surface Functionalization
- High-throughput polymer synthesis and nanoparticle fabrication is carried out following the same protocol and robotic set up described by Petersen et al19. The copolymer systems used for particle fabrication are based on sebacic acid (SA) and 1,6-bis(para-carboxyphenoxy)hexane (CPH), and 1,8-bis(para-carboxyphenoxy)-3,6-dioxaoctane (CPTEG) and CPH. A schematic representation of the robotic deposition apparatus utilized is presented in Figure 1b.
- Following nanoparticle fabrication, the holder containing tubes with the nanoparticle library is reattached to the linear actuator stage.
- For attachment of carbohydrates to the surface of polyanhydride particles, an amine-carboxylic acid coupling reaction 20 consisting of two consecutive reactions is performed.
- For the first reaction, the syringe in the first programmable syringe pump is filled with 10 equivalents (eq.) (equivalents of average molar carboxylic acid concentration on particle surface) of 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC) and 10 eq. of ethylenediamine in an aqueous solution, while the syringe in the second programmable syringe pump is loaded with 12 eq. of N-hydroxysuccinimide (NHS) in aqueous solution.
- Using the LabVIEW program, reagent suspensions are deposited into the nanoparticle library*.
- Next, each sample is sonicated (30 s at 40 Hz) and the tube holder is detached from the robotic platform.
- Nanoparticle suspensions are incubated for 9 h** with constant rotation at 4 °C.
- After reaction time is completed, tubes are centrifuged (12000 x g for 5 min) and returned to the robotic station to perform two washing steps.
- For washing, a syringe remains empty and loaded in the first programmable syringe pump while the syringe in the second syringe pump is filled with cold water. The supernatant in each tube is withdrawn into the empty syringe and the second pump deposits cold water.
- Homogenization of nanoparticle suspension is performed as described on step 2.6. Tubes are then centrifuged (12000 x g for 5 min) and a second wash step is performed as described on step 2.9.
- For the second reaction, two deposition steps are used. In the first deposition step, 12 eq. of EDC are loaded with one pump and 12 eq. of NHS are loaded with the second pump.
- The second deposition step includes 10 eq. of an specific saccharide on the first and second pumps (i.e., galactose, lactose or di-mannose)*** and a third pump with 10 eq. of glycolic acid (used as control****).
- Nanoparticle suspensions is homogenized as described on step 2.6 and incubated for 9 h with constant rotation at 4 °C.
- After the time of reaction is completed, a wash step is performed as described in steps 2.8, 2.9 and 2.10.
- The functionalized nanoparticle library is then placed in a vacuum chamber to dry for at least 2 hr.
- The functionalized nanoparticles are then characterized by X-ray photoelectron spectroscopy and a high throughput phenol-sulfuric acid assay to determine the surface composition and concentration of the saccharide respectively. Scanning electron microscopy and dynamic light scattering are utilized to determine particle size, size distribution, and surface charge.
Notes: *Deposition volumes vary with the mass of nanoparticles contained in each tube.
**Reaction times for first and second reactions can be changed to adjust the final saccharide concentration.
***Each saccharide is deposited into test tubes depending on the desired group.
****For the specific reaction employed in this study for the attachment of carbohydrates, glycolic acid is used as a linker control since deprotected saccharides already have this molecule covalently linked, which allows for further attachment to nanoparticle surface.
3. Representative Results
The fully protected dimannoside shown in Figure 2 was synthesized using the automation platform. The synthesized compound was characterized by 1H NMR in a VXR 400 MHz spectrometer using CDCl3 as solvent. The NMR spectrum is shown in Figure 3.
Utilizing the high-throughput nanoparticle fabrication and functionalization of polyanhydride nanoparticles described herein, attachment of dimannose, lactose and galactose has been carried out successfully 10, 11. Using this set up, optimal reaction conditions (i.e., reaction temperature and time) were identified to achieve desired nanoparticle functionalization and morphology. When the reaction was carried out at 4 °C instead of room temperature, a reduction in nanoparticle aggregation was observed by SEM (data not shown). Table 1 shows representative results of the characterization of functionalized 50:50 CPTEG:CPH nanoparticles with either di-mannose or lactose, synthesized at 4 °C. The data indicate a small increase in the average nanoparticle diameter due to the functionalization. While the non-functionalized nanoparticles had a negative zeta potential of approx. -20 mV, the functionalized particles showed a positive zeta potential value, demonstrating successful functionalization of the nanoparticle surface. Lactose and di-mannose are both neutral sugars; however, free amine groups from the ethylene diamine linker utilized to attach the saccharides may be responsible of the positive zeta potential.
Reaction time is another variable that could affect both the final morphology of the nanoparticles and the degree of sugar attachment achieved. By adjusting the reaction time, the final sugar concentration attached to the nanoparticles surface can be controlled as shown in Figure 4A. As expected, the concentration of dimannose on the surface of 50:50 CPTEG:CPH nanoparticles increased with the total time of reaction and reached a maximum after 18 hr. Nanoparticles functionalized with the 24 hr total reaction time were used to evaluate their ability to target CLRs on mouse bone-marrow derived dendritic cells (DCs). Flow cytometry was used to evaluate the expression of two CL receptors (i.e., CIRE (CD209, DC-SIGN) and mannose receptor (CD206)) after stimulation with non-functionalized, and lactose and di-mannose functionalized nanoparticles (Figure 4B). A higher expression of both receptors, which is an indicative of effective targeting, was obtained when cells were stimulated with both lactose and di-mannose functionalized nanoparticles. However, di-mannose-functionalized particles showed a higher level of expression indicating a specificity of this ligand for the receptors that were studied.
| Nanoparticle type | Average Particle Diameter (nm) | Average Particle ζ-Potential (mV) |
| Non-functionalized | 162 ± 43 | -20 ± 0.6 |
| Lactose | 235 ± 34 | 26 ± 2.4 |
| Di-mannose | 243 ± 32 | 30 ± 4.2 |
Table 1. Nanoparticle characterization. Non-functionalized and functionalized were characterized by quasi-elastic light scattering and zeta potential measurements. Particle size data represent the mean value ± standard deviation (SD) of dynamic light scattering data collected in three independent experiments. Zeta potential data represent the mean value ± SD of three independent readings. Change in the sign of the zeta potential demonstrates that sugar was efficiently conjugated to the 50:50 CPTEG:CPH nanoparticle surface.
Figure 1. (A) Graphical representation of the approach pursued with carbohydrate functionalization of polyanhydride nanoparticles and an example of the functionalized nanoparticle libraries that could be designed with the described high-throughput approach. (B) Schematic representation of the automated deposition apparatus utilized for particle functionalization, which consists of (i) three NE 1000 pumps; (ii) a robotic stage integrated by two actuators (Zaber): one for movement in the x direction and the other for movement in the y direction; (iii) a second robotic stage with two adjacent racks (appropriate for tubes and cuvettes) consisting of three actuators, one for each direction (x, y, and z). The pumps and a total of five actuators are connected in series. Actuators and pumps are operated by a computer using LabVIEW software. This diagram is not to scale. Click here to view larger figure.
Figure 2. Graphical representation of the automated iterative synthesis of carbohydrates using mannose as an example.
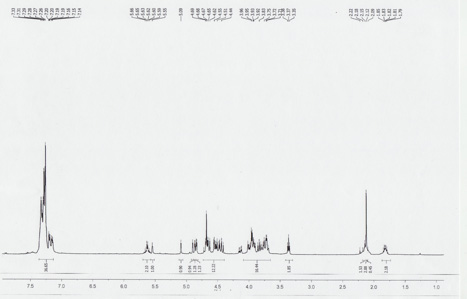
Figure 3. 1H NMR of the protected dimannoside.
Figure 4. (A) Effect of reaction time on nanoparticle surface concentration of saccharide. In the data shown, 50:50 CPTEG:CPH nanoparticles were functionalized with dimannose at different reaction times and the reaction was carried out at 4 °C. The average and standard error of two independent functionalization experiments is shown. (B) Lactose and di-mannose functionalized nanoparticles effectively target DC-SIGN (CIRE, CD209) and mannose receptor (CD206) on bone marrow-derived dendritic cells as demonstrated by the enhanced expression of these two markers after stimulation with functionalized 50:50 CPTEG:CPH nanoparticles when compared with the expression obtained with non-functionalized particles.
Discussion
The efficacy of carbohydrates as targeting agents to direct nanoparticle interactions to immune cells has been previously demonstrated 10, 11. Previous research in our laboratories have shown that specific sugars attached to polyanhydride nanoparticles are able to target different CLRs on antigen presenting cells (APCs), thereby enhancing the activation of immune cells which may be important for further T cell activation 10, 11. However, to achieve optimal targeting several parameters—su...
Disclosures
NLBP is cofounder and holds equity in the carbohydrate company LuCella Biosciences, Inc.
Acknowledgements
The authors would like to thank the U.S. Army Medical Research and Materiel Command (Grant # W81XWH-10-1-0806) and the National Institutes of Health (Grant # U19 AI091031-01 and Grant # 1R01GM090280) for financial support. BN acknowledges the Balloun Professorship in Chemical and Biological Engineering and NLBP acknowledges the Wilkinson Professorship of Interdisciplinary Engineering. We thank Julia Vela for her assistance in performing the nanoparticle functionalization experiments.
Materials
| Name | Company | Catalog Number | Comments |
| Name | Company | Catalog number | |
| Motorized XYZ Stage: 3x T-LSM050A, 50 mm travel per axis | Zaber Technologies | T-XYZ-LSM050A-KT04 | |
| NE-1000 Single Syringe Pump | New Era Pump Systems | NE-1000 | |
| Pyrex* Vista* Rimless Reusable Glass Culture Tubes | Corning | 07-250-125 | |
| ASW 1000 | Chemspeed Technologies | ||
| LabVIEW | National Instruments | 776671-35 | |
| SGE Gas Tight Syringes, Luer Loc | Sigma Aldrich | 509507 | |
| XL-2000 Sonicator | Qsonica | Q55 | |
| Mini-tube rotator | Fisher Scientific | 05-450-127 |
References
- Zepp, F. Principles of vacine design-lessons from nature. Vaccine. 28, C14-C24 (2010).
- Ulery, B. D., Phanse, Y., Sinha, A., Wannemuehler, M. J., Narasimhan, B., Bellaire, B. H. Polymer chemistry influences monocytic uptake of polyanhydride nanospheres. Pharm. Res. 26, 683-690 (2009).
- Torres, M. P., Wilson-Welder, J. H., Lopac, S. K., Phanse, Y., Carrillo-Conde, B., Ramer-Tait, A. E. Polyanhydride microparticles enhance dendritic cell antigen presentation and activation. Acta Biomater. 7, 2857-2864 (2011).
- Torres, M. P., Determan, A. S., Anderson, G. L., Mallapragada, S. K., Narasimhan, B. Amphiphilic polyanhydrides for protein stabilization and release. Biomaterials. 28, 108-116 (2007).
- Petersen, L. K., Ramer-Tait, A. E., Broderick, S. R., Kong, C. S., Ulery, B. D., Rajan, K. Activation of innate immune responses in a pathogen-mimicking manner by amphiphilic polyanhydride nanoparticle adjuvants. Biomaterials. 32, 6815-6822 (2011).
- Petersen, L. K., Xue, L., Wannemuehler, M. J., Rajan, K., Narasimhan, B. The simultaneous effect of polymer chemistry and device geometry on the in vitro activation of murine dendritic cells. Biomaterials. 30, 5131-5142 (2009).
- Lopac, S. K., Torres, M. P., Wilson-Welder, J. H., Wannemuehler, M. J., Narasimhan, B. Effect of polymer chemistry and fabrication method on protein release and stability from polyanhydride microspheres. J. Biomed. Mater. Res. B. 91, 938-947 (2009).
- Determan, A. S., Wilson, J. H., Kipper, M. J., Wannemuehler, M. J., Narasimhan, B. Protein stability in the presence of polymer degradation products: Consequences for controlled release formulations. Biomaterials. 27, 3312-3320 (2006).
- Determan, A. S., Lin, V. S. Y., Nilsen-Hamilton, M., Narasimhan, B. Encapsulation, stabilization, and release of BSA-FITC from polyanhydride microspheres. J. Controlled Release. 100, 97-109 (2004).
- Chavez-Santoscoy, A., Roychoudhury, R., Ramer-Tait, A. E., Pohl, N. L. B., Wannemuehler, M. J., Narasimhan, B. Tailoring the immune response of alveolar macrophages by targeting different C-type lectin receptors using "pathogen-like" amphiphilic polyanhydride nanoparticles. Biomaterials. , (2011).
- Carrillo-Conde, B., Song, E. -. H., Chavez-Santoscoy, A., Phanse, Y., Ramer-Tait, A., Pohl, N. L. Mannose-functionalized "pathogen-like" polyanhydride nanoparticles target C-type lectin receptors on dendritic cells. Mol. Pharmaceutics. 8, 1877-1886 (2011).
- Carrillo-Conde, B., Schiltz, E., Torres, M. P., Yu, J., Phillips, G., Minion, C. Amphipilic polyanhydrides for stabilization of Yersinia pestis antigens. Acta. Biomater. 6, 3110-3119 (2010).
- Reddy, S. T., Swartz, M. A., Hubbell, J. A. Targeting dendritic cells with biomaterials: developing the next generation of vaccines. Trends Immunol. 27, 573-580 (2006).
- Higashi, N., Fujioka, K., Denda-Nagai, K., Hashimoto, S., Nagai, S., Sato, T. The macrophage C-type lectin specific for galactose/N-acetylgalactosamine is an endocytic receptor expressed on monocyte-derived immature dendritic cells. J. Biol. Chem. 277, 20686 (2002).
- Geijtenbeek, T. B. Signalling through C-type lectin receptors: shaping immune responses. Nat. Rev. Immunol. 9, 465-479 (2009).
- Seeberger, P. H. Automated oligosaccharide synthesis. Chem. Soc. Rev. 37, 19-28 (2008).
- Seeberger, P. H. Automated Carbohydrate Synthesis as Platform to Address Fundamental Aspects of Glycobiology-Current Status and Future Challenges. Carb. Res. 343, 1889-1896 (2008).
- Jaipuri, F. A., Pohl, N. L. Toward solution-phase automated iterative synthesis: fluorous-tag assisted solution-phase synthesis of linear and branched mannose oligomers. Org. Biomol. Chem. 6, 2686-2691 (2008).
- Petersen, L. K., Chavez-Santoscoy, A., Narasimhan, B. Combinatorial synthesis of and high-throughput protein release from polymer film and nanoparticle libraries. J. Vis. Exp. , (2011).
- Song, E. -. H., Osanya, A. O., Petersen, C. A., Pohl, N. L. B. Synthesis of multivalent tuberculosis and Leishmania-associated capping carbohydrates reveals structure-dependent responses allowing immune evasion. J. Am. Chem. Soc. 132, 11428-11430 (2010).
- Hakamori, S. Aberrant glycosylation in tumor and tumor associated carbohydrate antigens. Adv. Cancer Res. 59, 257-331 (1989).
- Atherton, T., Sheppard, R. C. . Solid-phase peptide synthesis: a practical approach. , (1999).
- Caruthers, M. H. Gene synthesis machines: DNA chemistry and the uses. Science. 230, 281-285 (1985).
- Plante, O. J., Palmacci, E. R., Seeberger, P. H. Automated solid- phase synthesis of oligosaccharides. Science. 291, 1523-1527 (2001).
- Ko, K. -. S., Park, G., Yu, Y., Pohl, N. L. Protecting group-based colorimetric monitoring of fluorous-phase and solid-phase synthesis of oligoglucosamines. Org. Lett. 10, 5381-5384 (2008).
- Pohl, N. L., Chen, X. H. R., Wang, G. P. Automated solution-phase oligosaccharide synthesis and carbohydrate microarrays: development of fluorous-based tools for glycomics. Chemical Glycobiology. , 272-287 (2008).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved