Method Article
Evaluation of Muscle Function of the Extensor Digitorum Longus Muscle Ex vivo and Tibialis Anterior Muscle In situ in Mice
In This Article
Summary
Changes in limb muscle contractile and passive mechanical properties are important biomarkers for muscle diseases. This manuscript describes physiological assays to measure these properties in the murine extensor digitorum longus and tibialis anterior muscles.
Abstract
Body movements are mainly provided by mechanical function of skeletal muscle. Skeletal muscle is composed of numerous bundles of myofibers that are sheathed by intramuscular connective tissues. Each myofiber contains many myofibrils that run longitudinally along the length of the myofiber. Myofibrils are the contractile apparatus of muscle and they are composed of repeated contractile units known as sarcomeres. A sarcomere unit contains actin and myosin filaments that are spaced by the Z discs and titin protein. Mechanical function of skeletal muscle is defined by the contractile and passive properties of muscle. The contractile properties are used to characterize the amount of force generated during muscle contraction, time of force generation and time of muscle relaxation. Any factor that affects muscle contraction (such as interaction between actin and myosin filaments, homeostasis of calcium, ATP/ADP ratio, etc.) influences the contractile properties. The passive properties refer to the elastic and viscous properties (stiffness and viscosity) of the muscle in the absence of contraction. These properties are determined by the extracellular and the intracellular structural components (such as titin) and connective tissues (mainly collagen) 1-2. The contractile and passive properties are two inseparable aspects of muscle function. For example, elbow flexion is accomplished by contraction of muscles in the anterior compartment of the upper arm and passive stretch of muscles in the posterior compartment of the upper arm. To truly understand muscle function, both contractile and passive properties should be studied.
The contractile and/or passive mechanical properties of muscle are often compromised in muscle diseases. A good example is Duchenne muscular dystrophy (DMD), a severe muscle wasting disease caused by dystrophin deficiency 3. Dystrophin is a cytoskeletal protein that stabilizes the muscle cell membrane (sarcolemma) during muscle contraction 4. In the absence of dystrophin, the sarcolemma is damaged by the shearing force generated during force transmission. This membrane tearing initiates a chain reaction which leads to muscle cell death and loss of contractile machinery. As a consequence, muscle force is reduced and dead myofibers are replaced by fibrotic tissues 5. This later change increases muscle stiffness 6. Accurate measurement of these changes provides important guide to evaluate disease progression and to determine therapeutic efficacy of novel gene/cell/pharmacological interventions. Here, we present two methods to evaluate both contractile and passive mechanical properties of the extensor digitorum longus (EDL) muscle and the contractile properties of the tibialis anterior (TA) muscle.
Protocol
1. Evaluation of the Contractile and Passive Properties of the EDL Muscle Ex vivo
The contractile and passive properties of the EDL muscle are measured ex vivo using the Aurora Scientific in vitro muscle test system. Refer to Table 1 for materials and equipment.
1.1 Equipment preparation
- Assemble the tissue-organ bath by securing the oxytube to the water-jacket tissue bath. Attach the assembled bath to the muscle mounting apparatus. Connect the gas line to the oxytube. Fasten the water circulation lines to the water-jacket tissue bath and place the needle valve into the bath drainage.
- Turn on the circulating water-bath and adjust the temperature to 30 °C 7. Allow 5 PSI (pounds per square inch) of 95% O2-5% CO2 to flow through the oxytube. Fill the bath with Ringer's buffer. Equilibrate the buffer for at least 10 min with a steady gas flow by adjusting the oxytube valve.
- Turn on the instruments (stimulator, dual-mode lever system, and signal interface). Load the dynamic muscle control (DMC) software according to manufacturer's instruction.
1.2 EDL muscle dissection
All animal studies must be approved by the Institutional Animal Care and Use Committee.
- Anesthetize the mouse with intraperitoneal injection of 2.5 μl/g body weight of the anesthetic cocktail (refer to materials section). Throughout the surgical procedure, the depth of sedation was checked by performing a toe pinch. A supplement of 10% of the initial anesthetic dose is administrated when needed to keep the animal under anesthesia. Shave the hind limb. Maintain the body core temperature at 37 °C prior to dissection procedure by placing the mouse on a heating pad. The body temperature is monitored by constantly measuring the rectal temperature using a thermal probe.
- Position the mouse supine on the dissection board (Figure 1). Peel off the leg skin to expose the hind limb muscles. Secure the leg on the sylgard block using two dressmaker pins, one in the foot and the other in the gracilis muscle. Place a heat lamp above the mouse body to maintain the core body temperature at 37 °C. Constantly superfuse all exposed muscles with Ringer's buffer. Drain excess buffer through a vacuum line.
- Expose the distal TA tendon and the extensor ligament under a stereomicroscope by dissecting the skin toward the foot. Gently remove the fascia covering the TA muscle. Cut the extensor ligament to release the distal TA tendon.
- Cut the distal TA tendon and use it to peel off the TA muscle. Carefully remove the TA muscle at its proximal attachment. Place a thin piece of Ringer's buffer soaked cotton next to the EDL muscle to absorb bleeding caused by the rupture of the TA muscle vasculature. Use the vacuum line to remove excess buffer and blood.
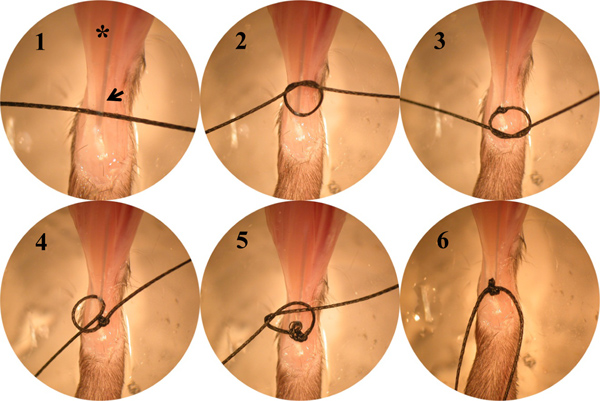
- Tie a double square knots followed by a loop knot using a bread silk suture at the muscle tendon junction (MTJ) of the distal EDL muscle (Figure 2). Make an incision in the distal portion of the biceps femoris muscle to expose the proximal EDL muscle. Repeat the same set of knots (Figure 2) at the MTJ of the proximal EDL tendon. Attach the lever arm hook to either the proximal or the distal knots with a double square knot using the same suture line. Cut off the remaining suture line.
- Cut the proximal EDL tendon superior to the proximal suture knot. Lift up the EDL muscle with the hook and cut the vasculature beneath the muscle. Cut the distal EDL tendon inferior to the distal suture knot to remove the EDL muscle from the hind limb. Cover the exposed hind limb with a piece of Ringer's buffer soaked cotton.
- Attach the hook to the lever arm. Align the muscle vertically between two electrodes. Secure the distal suture line to the fixed post. Lift up the tissue bath to submerge the muscle in Ringer's buffer. Adjust the resting tension to 1.0 g using the dual coarse/fine translation stage and allow the muscle to equilibrate for at least 10 min.
1.3 Measuring the contractile and passive properties of the EDL muscle
Use Table 2 to set up the parameters in the DMC software for each of the following measurements. Analyze the data using the dynamic muscle analysis (DMA) software.
1.3.1 Measuring the contractile properties of the EDL muscle
- Stimulate the EDL muscle three times at 150 Hz with 60 sec apart to stabilize the muscle 8.
- Stimulate the EDL muscle at different resting tensions to determine the optimal length (Lo). The optimal length is the length at which muscle develops maximum twitch tension. Allow the muscle to relax for 2 min.
- Adjust the resting tension to Lo. Measure muscle force at single twitch stimulation. Determine the absolute twitch force (Pt), time to peak tension (TPT) and half relaxation time (½ RT) of the Pt. Allow the muscle to relax for 2 min.
- Adjust the resting tension to Lo. Measure tetanic muscle force generated at different stimulation frequencies (50, 80, 100, 120, 150 and 200 Hz). Determine the absolute maximal tetanic muscle force (Po) where muscle force reaches the maximal. Measure the TPT and ½ RT of the Po 9.
- Allow the muscle to relax for 5 min. Adjust the resting tension to Lo. Apply 10 cycles of eccentric contractions with 2 min rest between cycles. Calculate the relative force loss of the Po after each cycle of eccentric contraction.
- Detach the EDL muscle from the apparatus and cut the tendons at the suture site. Determine the muscle wet weight and calculate the muscle cross sectional area (CSA) 6,10.
1.3.2 Measuring the passive properties of the EDL muscle
- Dissect the contralateral EDL muscle and attach it to the apparatus as described in Section 1.2, steps 2 to 7.
- Subject the EDL muscle to a six-step stretching protocol where the muscle is strained to 160% Lo with an increment of 10% Lo. Analyze the stress-strain profile 6.
- Evaluate the viscous property of the EDL muscle by measuring the stress relaxation rate (SRR) at the following time frames after stretching and holding the muscle at 10% Lo: from peak to 0.1s post-peak (pp), from 0.1 to 0.2s pp, from 0.2 to 0.5s pp, from 0.5 to 1s pp and from 1 to 1.5s pp.
- At the end of study, euthanize the mouse by cervical dislocation and/or decapitation while the mouse is still under anesthesia. Detach the EDL muscle from the apparatus and cut the tendons at the suture site. Determine the muscle wet weight and calculate the muscle cross sectional area (CSA) 6,10.
2. Evaluation of the Contractile Properties of the TA Muscle In situ
The contractile properties of the TA muscle are measured using the Aurora Scientific in situ muscle test system. Refer to Table 1 for materials and equipment.
2.1 Equipment preparation
- Heat up the thermo-controlled animal stage to 37 °C using the circulating water-bath.
- Turn on the instruments (stimulator, dual-mode lever system, and signal interface). Load the DMC software according to manufacturer's instruction.
2.2 Preparation of the TA muscle for in situ force measurement
- Anesthetize the mouse, shave the hind limb and expose the TA muscle as described in steps 1 to 3 in Section 1.2.
- Tie a double square knot around the patella ligament using a bread silk suture. Tie a double square knot followed by a loop knot at the MTJ of the distal TA muscle (Figure 2), tie another double square knot leaving a ~10 mm loop from the distal TA tendon knot using the same suture line. Place the second double square knot on the side of the loop.
- Remove the pins from the hind limb and position the animal prone. Expose the biceps femoris muscle. Make an incision in the midline to reveal the sciatic nerve. Tie a double square knot around the proximal end of the sciatic nerve. Trim one side of the suture lines and cut the nerve superior to the knot. Gently, pull the sciatic nerve toward the knee using the suture line and clear the surrounding connective tissue to free ~ 5 mm of its length. Do not stretch the nerve during this procedure and constantly superfuse the nerve with ringer buffer.
- Prepare the contralateral TA muscle as described in steps 1 to 3. Cover one of the exposed hind limb with a piece of Ringer's buffer soaked cotton. Constantly superfuse both hind limbs with pre-warmed (37 °C) Ringer's buffer. Remove excess buffer through a vacuum line.
- Position the animal prone on the animal platform. Attach the knee clamp holder to the animal platform and secure both knees to the metal pin with double square knots using the patella ligament suture lines. Pin both feet on the sylgard block using dressmaker pins. Secure the animal platform onto the thermo-controlled stage. Position the heat lamp to maintain the animal core body temperature at 37 °C.
- Secure the electrode holder to the animal platform and lay the sciatic nerve on the electrode using the suture line. Keep the electrode away from the hind limb muscles. Cut the distal TA tendon of the uncovered hind limb at the MTJ suture site. Attach the distal TA tendon suture loop to the lever arm hook. Cover the exposed hind limb muscle with a warm Ringer's buffer soaked cotton.
2.3 Measuring the contractile properties of the TA muscle
- Use Table 2 to set the parameters in the DMC software. Follow the same protocol described in Section 1.3.1 to determine the contractile properties of the TA muscle. Analyze the data using the DMA software.
- After contractile property measurement, detach the distal TA tendon suture loop from the leveler arm hook. Remove the TA muscle. Determine the muscle wet weight and calculate the CSA 10.
- Measure the contractile properties of the contralateral TA muscle according to steps 1 to 3 described above. Euthanize the mouse according to institutional guidelines at the end of the study.
Results
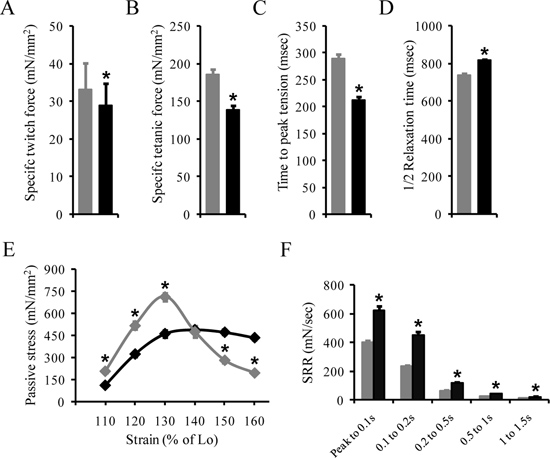
The following results are a representation from our previous reports 6,9. Data are presented as mean±standard error of mean. Table 3 shows the morphometric properties of the EDL muscle in normal BL10 and dystrophin-deficient (mdx) mice at 4 to 6 months of age. Figure 4 shows representative contractile and passive properties of the EDL muscle from BL10 and mdx mice. The contractile properties of the EDL muscle are described by the following terms including the specific (absolute force divided by the CSA) twitch force (Figure 4A), specific maximal tetanic force (Figure 4B), TPT and ½ RT of the absolute maximal tetanic force (Figure 4C and D). The TPT and ½ RT can also be calculate from the absolute twitch force. The stress-strain profile (Figure 4E) and SRR (Figure 4F) are used to describe the passive properties of the EDL muscle.
Absence of dystrophin has a significant impact on the contractile and passive properties of the EDL muscle 6,9. Specific twitch and tetanic forces are significantly reduced in the mdx EDL muscle. The TPT is significantly faster while the ½ RT is significantly slower in the mdx EDL muscle. The stress-strain profile suggests that stiffness is significantly increased in the mdx EDL muscle. The mdx EDL muscle also yields a significantly much higher resistance force (passive stress) before reaching the peak stress, while the post-peak stresses decline much faster. Further, the SRR was significantly higher in the mdx EDL muscle compared to that of the BL10 EDL muscle.
Statistical analysis
Statistical significance between two groups is analyzed by the Student t-test. For statistical significance among multiple groups, One-way or Two-way ANOVA analysis followed by Bonferroni post hoc analysis is recommended using the SAS software (SAS Institute Inc., Cary, NC). Difference is considered significant when p < 0.05.
Table 1. Materials and equipment.
| Experiment | Resting tension (gram) | Pulse frequency (Hz) | Pulse width (ms) | Stimulation duration (ms) | Stretch length | Stretch duration (ms) | Stretch rate | Comments |
| 1. Evaluation of contractile and passive properties of the EDL muscle ex vivo | ||||||||
| 1.3.1 Measuring the contractile properties of the EDL muscle | ||||||||
| 1. Warm up | 1.0 | 150 | 0.2 | 300 | Rest the muscle for 60 sec between each stimulus. These preliminary tetanic contractions stabilize the muscle for subsequent measurements. | |||
| 2. Optimal muscle length (Lo) | 0.5, 1.0, 1.5 and 2.0 | 1 | 0.2 | 300 | Allow the muscle to relax for 30 sec between each stimulus. Measure the muscle optimal length using a digital caliper. | |||
| 3. Single twitch force (Pt) | Adjust resting tension to Lo | 1 | 0.2 | 300 | ||||
| 4. Tetanic muscle force | Adjust resting tension to Lo | 50, 80, 100, 120, 150 and 200 | 0.2 | 300 | Allow the muscle to relax for 1 min between each stimulus. Determine the frequency that generate the maximal absolute tetanic force (Po). | |||
| 5. Eccentric contraction | Adjust resting tension to Lo | Use the frequency that generates the maximum tetanic force (Po) | 0.2 | 700 | 10% Lo | last 200 ms of the stimulation duration | 0.5 Lo/sec | Repeat the eccentric contraction for 10 cycles with 2 min rest between cycles. |
| 6. CSA of the EDL muscle | CSA = (muscle mass (g) / [1.06 g/cm3 x (Lo x 0.44)]. 1.06 g/cm3 is the muscle density and 0.44 is the EDL muscle fiber length to Lo ratio. | |||||||
| 1.3.2 Measuring the passive properties of the EDL muscle | ||||||||
| 1. Six-step stretching protocol | Adjust resting tension to Lo | 10% Lo | 2 cm/sec | Repeat the stretching protocol with an increment of 10% Lo till 160% Lo is reached. Alow 1.5 sec between stretch cycles. | ||||
| 2. SRR | Adjust resting tension to Lo | 10% Lo | 2 cm/sec | SSR is calculated by dividing the difference in the stress with the time elapsed between two time points in a time frame. | ||||
| 2.3 Measuring the contractile properties of the TA muscle | ||||||||
| 1. Warm up | 4.0 | 150 | 0.2 | 300 | Rest the muscle for 60 sec between each stimulus. | |||
| 2. Optimal muscle length (Lo) | 3.0, 4.0, 5.0, 6.0 and 7.0 | 1 | 0.2 | 300 | Allow the muscle to relax for 30 sec between each stimulus. Measure the muscle optimal length using a digital caliper. | |||
| 3. CSA of the TA muscle | CSA = (muscle mass (g) / [1.06 g/cm3 x (Lo x 0.6)]. 0.6 is the TA muscle fiber length to Lo ratio. | |||||||
Table 2. Parameters for the evaluation of the mechanical properties of the EDL and TA muscles.
| Strain | Age (month) | Body weight (g) | EDL weight (mg) | EDL Lo (mm) | EDL CSA (mm2) |
| BL10 | 6 | 32.03 ± 0.57 | 13.90 ± 0.77 | 14.09 ± 0.04 | 2.12 ± 0.12 |
| mdx | 6 | 35.44 ± 0.42* | 16.73 ± 0.42* | 13.93 ± 0.05* | 2.57 ± 0.07* |
Table 3. Morphometric properties of the EDL muscle. *, the value in mdx mice is significantly different from that of age-matched BL10 mice.
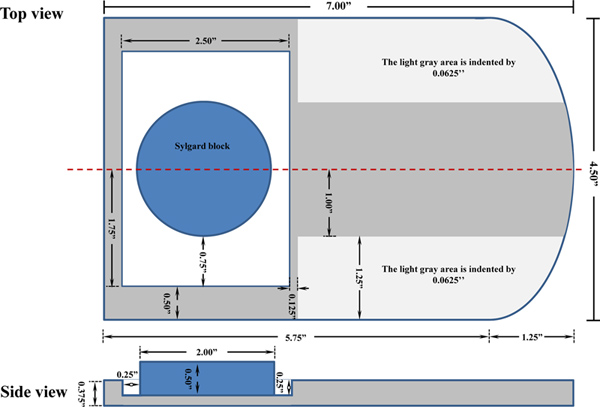
Figure 1. A schematic diagram of the custom-made mouse dissection board. The dissection board is made from a ½ inch thick plexiglass and was fabricated at the institutional shop. Click here to view larger figure.
 />
/>
Figure 2. A series of digital images showing the steps of tying a double square knot followed by a loop knot at the MTJ. Asterisk, the EDL muscle; Arrow, the distal tendon of the EDL muscle.
 />
/>
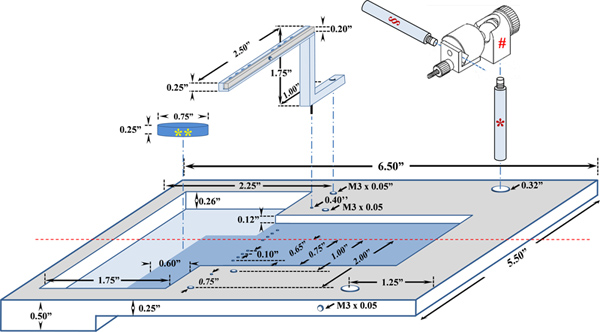
Figure 3. A schematic diagram of the custom-made platform for in situ TA muscle function assay. The plexiglass animal platform and the stainless steel knee holder were designed to mount on the 809B in situ mouse apparatus. *, Stainless steel rod (Cat# MPR-2.0, Siskiyou, Grants Pass, OR); #, Universal electrode holder (Cat# MXB, Siskiyou, Grants Pass, OR); §, electrode attachment rod (Cat# MPR-3.0, Siskiyou, Grants Pass, OR); **, Sylgard block. Click here to view larger figure.
 />
/>
Figure 4. Representative results for the contractile and passive properties of the EDL muscle. The contractile properties of the EDL muscle are characterized by the specific twitch force (A), the specific tetanic force (B), the time to peak tension (C) and the half relaxation time (D). The passive properties of the EDL muscle are assessed by the stress strain profile (E) and the SSR. *, mdx mice are significantly different from age-matched BL10 mice.
Discussion
In this protocol, we have illustrated physiological assays for measuring the contractile and passive properties the EDL muscle and the contractile properties of the TA muscle. A major concern in muscle physiology studies is the oxygenation of the target muscle. For large muscles (such as the TA muscle), the in situ approach is preferred because oxygen diffusion from Ringer's buffer may not reach the center of the muscle in an in vitro assay. In situ approach does not disturb normal blood supply and hypoxia-associated artificial effects are avoided. The EDL muscle is one of the most commonly used muscle in physiology study. Adequate oxygenation of the entire muscle can be achieved in an in vitro system because of the small size of the muscle. Further, the in vitro system provides an enclosed environment to manipulate the concentrations of ions (Ca 2+, Na+ and K+) and chemicals (ATP and glucose) that are necessary for optimal muscle force generation. This offers a great opportunity to study the effect of these variables on force production.
Accurate measurement of the contractile and passive properties of the limb muscle is critical to study skeletal muscle function. Characteristic changes of these properties are often considered as the hallmarks of various muscle diseases. Changes in these parameters are also important indicators to determine whether an experimental therapy is effective or not.
Disclosures
Open access fees have been paid for by Aurora Scientific.
Acknowledgements
This work was supported by grants from the National Institutes of Health (AR-49419, DD), Muscular Dystrophy Association (DD), and NIH training grant T90DK70105 (CH).
Materials
| Name | Company | Catalog Number | Comments |
| Material | Manufacturer | Specifications and comments | |
| Tissue-organ bath | Radnoti LLC, CA, USA | Water-jacket tissue bath (Cat #158351-LL), Oxygen disperser tube (Cat #160192), Luer valve (Cat#120722) | |
| Circulating water bath | Fisher Scientific, Waltham, MA, USA | ||
| Gas mix | Airgas National, Charlotte, NC, USA | 95% O2 and 5% CO2 | |
| In vitro muscle function assay apparatus | Aurora Scientific, Aurora, ON, Canada | The system consists of a stimulator (Model# 701A), a dual-mode lever system (Model#300C or 305C), a signal interface (Model # 604B) and a test apparatus (Model# 800A) to vertically mount tissue organ bath | |
| In vitro muscle function assay software | Dynamic muscle control (DMC) software and dynamic muscle control data analysis (DMA) software | ||
| Mouse anesthesia cocktail mixed in 0.9% NaCl | Refer to the institutional guidelines | Ketamine (25 mg/ml), xylazine (2.5 mg/ml) and acepromazine (0.5 mg/ml). Throughout the surgical procedure, a supplement of 10 % of the initial dose may be needed to keep animal under anesthesia. | |
| Sylgard | World Precision Instrument | Cat#SYLG184 | |
| A custom-made Plexiglas dissection board | In house designed | Refer to Figure 1 | |
| Heating lamp | Tensor Lighting Company, Boston, MA, USA | 15 Watt lamp to keep the mouse warm during dissection | |
| Ringer's Buffer | Chemicals are purchased from Fisher Scientific, Waltham, MA, USA | Composition in mM: 1.2 NaH2PO4 (Cat#S369) , 1 MgSO4 (Cat# M63), 4.83 KCl (Cat# P217), 137 NaCl (Cat# 217), 24 NaHCO3 (Cat# S233), 2 CaCl2 (Cat #C79) and 10 glucose (Cat# D16). Dissolve chemicals individually and mix in the order listed above. Store at 4 °C. | |
| Stereo dissecting microscope | Nikon, Melville, NY, USA | ||
| Dissection tools | Fine Science Tools, Foster City, CA, USA | Coarse forceps, coarse scissors, fine forceps (Straight and 45 ° angle) | |
| Braided silk suture #4-0 | SofSilk USSC Sutures, Norwalk, CT, USA | Cat # SP116 | |
| A custom-made stainless steel hook | Small Parts, Inc. | 2'' long S/S 304V (0.18'' diameter) for force transducer 305C or 2.5'' long S/S 304V (0.012'' diameter) for transducer 300C (Cat# ASTM A313) | |
| In situ muscle function assay system | Aurora Scientific, Aurora, ON, Canada | The system (809B, in situ mouse apparatus) consist of a stimulator (Model# 701B), a dual-mode lever system (Model# 305C), a signal interface (Model# 604A) and a thermo controlled footplate apparatus (Model# 809A) | |
| In vitro muscle function assay software | Aurora Scientific, Aurora, ON, Canada | Dynamic muscle control (DMC) software and dynamic muscle control data analysis (DMA) software | |
| A custom-made TA assay animal platform | In house designed | Refer to Figure 2 | |
| A custom-made stainless steel hook | Small Parts, Inc. | Cat# ASTM A313 | 0.5'' long S/S 304V (0.18'' diameter) |
| Custom-made 25G platinum electrodes | Chalgren Enterprises, Gilroy,CA | Solder two 0.016'' thick platinum wires to two 24G electric wires |
Table 1. Materials and equipment.
References
- Huijing, P. A. Muscle as a collagen fiber reinforced composite: a review of force transmission in muscle and whole limb. J. Biomech. 32, 329-345 (1999).
- Moss, R. L., Halpern, W. Elastic and viscous properties of resting frog skeletal muscle. Biophys. J. 17, 213-228 (1977).
- Hoffman, E. P., Brown, R. H., Kunkel, L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 51, 919-928 (1987).
- Petrof, B. J., Shrager, J. B., Stedman, H. H., Kelly, A. M., Sweeney, H. L. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc. Natl. Acad. Sci. U.S.A. 90, 3710-3714 (1993).
- Pastoret, C., Sebille, A. mdx mice show progressive weakness and muscle deterioration with age. J. Neurol. Sci. 129, 97-105 (1995).
- Hakim, C. H., Grange, R. W., Duan, D. The passive mechanical properties of the extensor digitorum longus muscle are compromised in 2- to 20-mo-old mdx mice. J. Appl. Physiol. 110, 1656-1663 (2011).
- Segal, S. S., Faulkner, J. A. Temperature-dependent physiological stability of rat skeletal muscle in vitro. Am. J. Physiol. 248, 265-270 (1985).
- Grange, R. W., Gainer, T. G., Marschner, K. M., Talmadge, R. J., Stull, J. T. Fast-twitch skeletal muscles of dystrophic mouse pups are resistant to injury from acute mechanical stress. Am. J. Physiol. Cell Physiol. 283, 1090-1101 (2002).
- Hakim, C. H., Duan, D. Gender differences in contractile and passive properties of mdx extensor digitorum longus muscle. Muscle Nerve. 45, 250-256 (2012).
- Hakim, C. H., Li, D., Duan, D. Monitoring murine skeletal muscle function for muscle gene therapy. Methods Mol. Biol. 709, 75-89 (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved