Method Article
The Sciatic Nerve Cuffing Model of Neuropathic Pain in Mice
In This Article
Summary
Neuropathic pain is a consequence of a lesion or disease affecting the somatosensory system. The “cuff model” of neuropathic pain in mice consists of the implantation of a polyethylene cuff around the main branch of the sciatic nerve. Mechanical allodynia is tested using von Frey filaments.
Abstract
Neuropathic pain arises as a consequence of a lesion or a disease affecting the somatosensory system. This syndrome results from maladaptive changes in injured sensory neurons and along the entire nociceptive pathway within the central nervous system. It is usually chronic and challenging to treat. In order to study neuropathic pain and its treatments, different models have been developed in rodents. These models derive from known etiologies, thus reproducing peripheral nerve injuries, central injuries, and metabolic-, infectious- or chemotherapy-related neuropathies. Murine models of peripheral nerve injury often target the sciatic nerve which is easy to access and allows nociceptive tests on the hind paw. These models rely on a compression and/or a section. Here, the detailed surgery procedure for the "cuff model" of neuropathic pain in mice is described. In this model, a cuff of PE-20 polyethylene tubing of standardized length (2 mm) is unilaterally implanted around the main branch of the sciatic nerve. It induces a long-lasting mechanical allodynia, i.e., a nociceptive response to a normally non-nociceptive stimulus that can be evaluated by using von Frey filaments. Besides the detailed surgery and testing procedures, the interest of this model for the study of neuropathic pain mechanism, for the study of neuropathic pain sensory and anxiodepressive aspects, and for the study of neuropathic pain treatments are also discussed.
Introduction
Neuropathic pain is usually chronic and arises as a consequence of a lesion or a disease affecting the somatosensory system. Maladaptive changes in injured sensory neurons and along the entire nociceptive pathway within the central nervous system participate in this complex syndrome. Various models have been developed in rodents for studying neuropathic pain and its treatments1-3.
Based on known etiologies, the models of neuropathic pain aim at mimicking the polyneuropathy observed in diabetes, the injuries to peripheral nerves, the central injuries, the trigeminal neuralgia, the neuropathies consecutive to chemotherapy, the post-herpetic neuralgia, etc. Different models of peripheral nerve injury in rodents focus on the sciatic nerve. These models depend on a compression and/or a section of this nerve. Indeed, the sciatic nerve affords relative easy surgery and allows for tests based on paw withdrawal reflexes. The models of chronic nerve compression include for example: the chronic constriction injury (CCI)4,5, the sciatic nerve cuffing6-9, the partial sciatic nerve ligation (PSL)10, the spinal nerve ligation (SNL)11, or the common peroneal nerve ligation12. Models referred to as "spared nerve injury" (SNI) are also widely used. They consist of a tight ligation and axotomy of two out of the three terminal branches of the sciatic nerve, while the third branch remains intact13-15. The various models of neuropathic pain, which target the sciatic nerve, result in a chronic mechanical allodynia (a nociceptive response to a normally non-nociceptive stimulus) on the injured hind paw.
Here, the detailed surgery procedure for the "cuff model" of neuropathic pain in mice is described. It consists in the implantation of a polyethylene cuff around the main branch of the sciatic nerve6-9. The use of von Frey filaments is also described. These filaments allow assessing the mechanical allodynia which is a long lasting nociceptive symptom present in this model.
Protocol
Protocols have been approved by the "comité d'éthique en matière d'expérimentation animale de Strasbourg" (CREMEAS).
1. Baseline Measurement of Paw Withdrawal Thresholds
- Allow the mice to habituate to the animal facilities for at least 10 days to 2 weeks before initiating the testing procedures.
- Habituate the mice to the von Frey testing set-up and to the von Frey procedure that are described in section 4.
- Before surgery, evaluate the mechanical paw withdrawal thresholds with von Frey filaments as described in the section 4.3. Note: Repeat the procedure on separate days until at least three stable consecutive values are obtained for paw withdrawal thresholds.
- Assign the mice to the different experimental groups so that these groups do not initially differ for paw withdrawal thresholds.
2. Surgery Procedure for Cuff Implantation
- Weigh the animal. Note: Mouse body weight should be over 20 g for the cuff insertion procedure described below.
- Anesthetize the animal with an intraperitoneal injection of 4 ml/kg of a mixture of ketamine (17 mg/ml) and xylazine (2.5 mg/ml) in 0.9% NaCl, which provides around 45 min of anesthesia.
- Check the absence of paw reflexes by pinching a hind paw with tweezers and check the absence of eye reflexes to make sure that the animal is fully anesthetized.
- Shave the right leg from the knee to the hip using an electrical shaver.
- Apply protective eye liquid gel to the eyes with a cotton-tipped swab.
- Place the animal on its left side and place the right hind limb on a small pillow and maintain the right hind limb to the pillow with adhesive tape.
- Disinfect the surgery field with chlorhexidine and 70% ethanol using gauze pad or cotton-tipped swab.
- Find the femur using the forefinger and make an incision of approximately 0.5 cm, parallel to the femur and approximately 1.5 mm anterior to the femur.
- Separate the muscles close to the femur with two autoclaved sticks. Notes: Never cut the muscle. Normally, the muscle layers separate easily without any bleeding and the sciatic nerve is then visible. In case of bleeding, use a sterile cotton-tipped swab to absorb the blood.
- Insert two autoclaved sticks below the sciatic nerve to expose its main branch, and hydrate the nerve with a sterile physiological solution (0.9% NaCl).
- Hold the pre-prepared sterile 2 mm section of split PE-20 polyethylene tubing (cuff), 0.38 mm ID / 1.09 mm OD, with the help of a pointed steel stick and a bulldog clamp.
- Insert the pointed steel stick into the cuff, which will slightly open it.
- Using the cuff lateral opening, insert the bulldog at one end of the cuff and parallel to the cuff. Rotate the bulldog (180°) so that it will hold the cuff by the side that is opposite to the lateral opening. Close the bulldog and remove the pointed steel stick. Note: The rotation is done to allow holding-on the cuff in an optimized position for the insertion, the bulldog clamp is also helping to maintain the cuff partly open. The model and the size of the bulldog clamp are critical for this step of the procedure.
- Have a second experimenter hold the two sticks under the nerve and gently separate the sticks to facilitate the access to a section of sciatic nerve that is around 4 mm long.
- Insert the 2 mm cuff around the main branch of the sciatic nerve, starting by inserting the part of the cuff that is distal to the bulldog around the part of the nerve that is proximal to the hip.
- Close the cuff gently by exerting pressure on its two distal sides with pliers, without squeezing or changing the form of the tube. Turn the cuff to ensure that it is closed correctly.
- Suture the shaved skin layer with surgical knots.
- Place the mouse on its left side in a clean home cage. Keep it under the heat lamp until the mouse is awake.
- Add extra water and place some chow directly in the home cage.
3. Surgery Procedure for Sham Controls
- Apply the same surgery procedure as described above from step 2.1 to step 2.9, then follow with steps 2.15 to 2.17. For sham controls, omit the steps 2.10 to 2.14 that only concern the cuff insertion.
4. von Frey Testing
- Place the mice in clear individual boxes (7 cm x 9 cm x 7 cm) with holes, on an elevated perforated plate of smooth stainless steel (1 m x 50 cm, 5 mm circle perforations with 2.5 mm between perforation borders). Note: Up to 12 mice can be concomitantly tested on this set-up. Operated animals can be tested the day after the surgery. However, 3 days of recovery are recommended to diminish the post-surgery hypersensitivity observed in sham controls.
- Allow the animals to habituate for 15 min prior to testing.
- Apply the von Frey filaments to the plantar surface of each hind paw in a series of ascending forces. Notes: The von Frey filaments are plastic hairs of calibrated diameters. They are 5 cm long and are fixed on hand-held applicators. The speed of filament application, the degree of bending and the duration of the application can influence the threshold values that are obtained with this test3. With the present procedure in mice, the filaments that are the most often used are the 0.16, 0.4, 0.6, 1, 1.4, 2, 4, 6, 8, and 10 g.
- Apply the chosen filament to the plantar surface of the left paw until the filament just bends. Repeat the procedure three to five consecutive times, and then do the same to the right paw. Once the filament has been tested on both paws, test the next animal. Notes: Avoid paw lateral borders which can be more sensitive. The expected response is a paw withdrawal, sudden flinching or paw licking. Consider the response as positive if at least three expected responses are observed out of five trials. A given paw is always tested three times, but the fourth and the fifth trials are done only if 1 or 2 response(s) was (were) observed during the first three tests. In C57BL/6J mice, start the pre-surgery tests with the 1.4 g filament. After surgery, start the tests with the 0.4 g filament. If a positive response is observed with the first tested filament, test a filament of lower force (instead of greater) at step 4.3.2.
- Apply the same filament to the next animals according to the 4.3.1 procedure. Once all animals are tested, start again on the first animal with the next filament of greater force. Repeat the procedure until all mice give a positive response. Notes: Test each animal until two consecutive filaments give a positive response. Consider the gram value of the lower filament that gave a positive response as the paw withdrawal threshold for this animal.
Results
The data are expressed as mean ± SEM. Statistical analyses were performed using multi-factor analysis of variance (ANOVA) or unpaired t-tests in accordance with the experimental design. For these analyses, the Sham and Cuff surgery groups as well as the saline vs. drug treatments were considered as between-group factors. When appropriate, repeated measure analyses were used for the time course data. The post-hoc comparisons were performed using the Duncan test. Statistical significance was considered at p<0.05.
When using the procedures that are described above, the cuff implantation results in an ipsilateral allodynia as illustrated in Figure 1. Once the mouse is habituated to the testing procedure, the values for paw withdrawal thresholds in the von Frey test remain stable over time and are not affected by the surgical procedure per se, as illustrated in Sham animals. It should however be noted that a transitory post-surgical allodynia can usually be observed in Sham mice. When such allodynia is present, the paw withdrawal response returns to baseline after a few days post-surgery. In Cuff mice, the ipsilateral allodynia is already present on the first days post-surgery and is maintained for more than 2 months (see 9, and Figure 1; F8,344=29.5, p<0.001). The cuff-induced allodynia remains ipsilateral in C57BL/6J mice when it is measured by the von Frey test as described above, but in other conditions a presence of allodynia on the contralateral paw can also be observed8. The absolute values for baseline are usually between 4 and 6 g in C57BL/6J mice, but the testing protocol may affect these values.
Tricyclic antidepressants are among clinical first-line treatments for neuropathic pain. In this model, the tricyclic antidepressant drug nortriptyline (5 mg/kg, intraperitoneal, twice a day) relieves the neuropathic allodynia after around 2 weeks of treatment, as illustrated in Figure 2 (F7,91=15.3, p<0.001; post-hoc: (CuffNor=Sham)>CuffSal at p<0.001 on days 29 - 34). At this dose, no acute analgesic action of the antidepressant is observed16,17. To mimic the lasting pain relief that is present in patients taking such drugs, the mice can be tested before the morning drug administration rather than after. Such procedure allows assessment of a long-lasting effect primed by previous days of treatment. In this case, it requires 1 to 2 weeks of treatment to observe a lasting relief of the neuropathic allodynia. When the treatment is interrupted, a relapse is usually observed within 3 to 4 days18. Beside some antidepressants, gabapentinoids are the other first-choice treatments for neuropathic pain. Gabapentin has an acute and transitory analgesic action in this model16, but it also displays a delayed and long-lasting antiallodynic action when testing the animal each day before the drug administration (Figure 3; p<0.001). This action is faster than with antidepressant drugs.
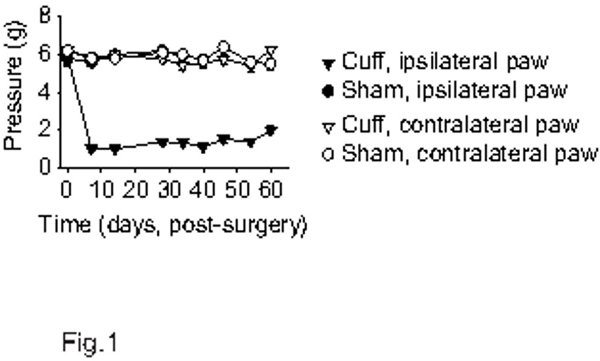
Figure 1. Mechanical paw withdrawal thresholds in the cuff model of neuropathic pain in mice. Adult male C57BL/6J mice were habituated to the von Frey procedure until a stable baseline was obtained (the baseline is represented at point 0 on the graph). Both paws were tested. The Cuff mice display ipsilateral mechanical allodynia as showed by the lowered paw withdrawal thresholds (n=10 per group).
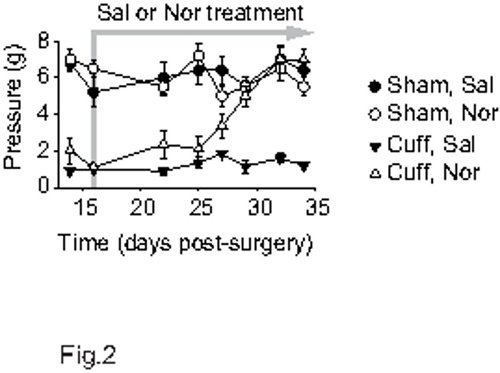
Figure 2. Delayed antiallodynic action of a tricyclic antidepressant. After two weeks post-surgery, mice received intraperitoneal treatment twice a day (morning and evening) with either 0.9% NaCl or 5 mg/kg nortriptyline hydrochloride (n=5 or 6 per group). The von Frey test was done before the morning treatment. With this procedure, a delayed antiallodynic action of nortriptyline is observed, which requires around 12 days of treatment.
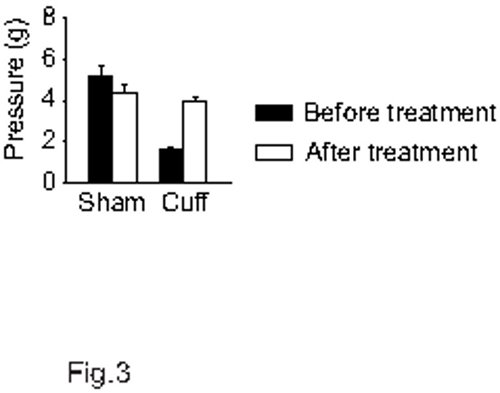
Figure 3. Antiallodynic action of a gabapentinoid. After three weeks post-surgery, mice received intraperitoneal treatment twice a day (morning and evening) with either 0.9% NaCl or 10 mg/kg gabapentin (n=5 per group). The von Frey test was done before the morning treatment. With this procedure, a delayed and lasting antiallodynic action of gabapentin is observed. Data are presented before starting the treatments and at the 6th day of treatments.
Discussion
The "cuff" model was initially developed in rats to obtain a standardized and reproducible chronic constriction injury with the implantation of multiple cuffs around the sciatic nerve6. It was then modified to implant a single cuff7,8, even though some research groups still use multiple cuff insertion19-22. It was then adapted to mice9,23, which opened the possibility to use transgenic animals. The cuff is usually 2 mm long, but other lengths have also been used in rats22. The polyethylene tubing depends on the species: PE-20 in mice9, and PE-6024,25 or PE-907,8,26,27 in rats.
The mechanical allodynia is measured with von Frey hairs. In this test, the absolute values for paw withdrawal thresholds may depend upon the surface on which the animal stands28 or upon the duration of filament bending3, but these factors do not affect the detection of the neuropathic allodynia.
The "cuff" model is of interest for the study of neuropathic pain mechanisms. It was used to study morphological changes in myelinated and unmyelinated fibers6,29, and functional changes in sensory neurons, primary afferents and spinal neurons19,21,22,30-35. It allowed demonstration that glial activation and a central shift in neuronal anion gradient participate in changes in the activity and in the responses of spinal nociceptive neurons and in neuropathic allodynia24,36-38. The influence of glutamate receptors7,39-41, of opioid receptors16,42-45 and of nicotinic receptors46 was also studied in this model.
Another interest of the model is its response to current treatments of neuropathic pain, i.e., gabapentinoids and antidepressants. Similar to clinical observations: gabapentinoids display both an acute short-lasting analgesic action at high dose and a delayed sustained relieving action that is observed after a few days of treatment, tricyclic antidepressants and selective serotonin and noradrenaline reuptake inhibitors have no acute analgesic effect at relevant dose but they display a delayed sustained relieving action that requires 1 to 2 weeks of treatment, and the selective serotonin reuptake inhibitor fluoxetine is ineffective16. The model is thus appropriate to study the molecular mechanism underlying these treatments16-18,44,45,47, which may reveal new therapeutic targets to test in patients48-51.
Lastly, the model also allows the study of the anxiodepressive consequences of neuropathic pain. Clinically, these consequences affect around a third of neuropathic pain patients but are preclinically less studied than the sensory aspects of pain. In this model, a time-dependent development of anxiety-like and depressive-like phenotypes is present52 and the related mechanism can thus be addressed.
The standardized cuffs and procedures in this mouse model of neuropathic pain result in low interindividual variability for the mechanical allodynia. The possibility to use genetically modified animals17,18,44-47,52, the long-lasting allodynia, the response to clinically used treatments and the time-dependent development of anxiodepressive symptoms make this model appropriate for the study of the various aspects and consequences of neuropathic pain and its treatments, which have already brought valuable information to this field of research.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgements
This work was supported by the Centre National de la Recherche Scientifique (contract UPR3212), the University of Strasbourg and by a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (to I.Y). Publication costs are supported by the Neurex network (Program Interreg IV Upper Rhine).
Materials
| Name | Company | Catalog Number | Comments |
| PE-20 polyethylene tubing | Harvard Apparatus | PY2-59-8323 | Splitted before surgery |
| Ketamine | Centravet | IMA004 | |
| Xylazine HCl | Sigma | X1251 | Freshly prepared before surgery |
| Ocry-gel | Centravet | ||
| Pliers | FST | 11003-12 | 52.5 mm straight |
| Bulldog clamp | FST | p130 18038-45 | |
| Perforated plate | CTTM | ||
| von Frey filaments | Bioseb | NC-12775 |
References
- Colleoni, M., Sacerdote, P. Murine models of human neuropathic pain. Biochim. Biophys. Acta. 1802, 924-933 (2010).
- Jaggi, A. S., Jain, V., Singh, N. Animal models of neuropathic pain. Fundam. Clin. Pharmacol. 25, 1-28 (2011).
- Barrot, M. Tests and models of nociception and pain in rodents. Neuroscience. 211, 39-50 (2012).
- Bennett, G. J., Xie, Y. K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 33, 87-107 (1988).
- Austin, P. J., Wu, A., Moalem-Taylor, G. Chronic constriction of the sciatic nerve and pain hypersensitivity testing in rats. J. Vis. Exp. (61), (2012).
- Mosconi, T., Kruger, L. Fixed-diameter polyethylene cuffs applied to the rat sciatic nerve induce a painful neuropathy: ultrastructural morphometric analysis of axonal alterations. Pain. 64, 37-57 (1996).
- Fisher, K., Fundytus, M. E., Cahill, C. M., Coderre, T. J. Intrathecal administration of the mGluR compound, (S)-4CPG, attenuates hyperalgesia and allodynia associated with sciatic nerve constriction injury in rats. Pain. 77, (1998).
- Pitcher, G. M., Ritchie, J., Henry, J. L. Nerve constriction in the rat: model of neuropathic, surgical and central. 83, 37-46 (1999).
- Benbouzid, M., et al. Sciatic nerve cuffing in mice: a model of sustained neuropathic pain. Eur. J. Pain. 12, 591-599 (2008).
- Seltzer, Z., Dubner, R., Shir, Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 43, 205-218 (1990).
- Kim, S. H., Chung, J. M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 50, 355-363 (1992).
- Vadakkan, K. I., Jia, Y. H., Zhuo, M. A behavioral model of neuropathic pain induced by ligation of the common peroneal nerve in mice. J. Pain. 6, 747-756 (2005).
- Decosterd, I., Woolf, C. J. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 87, 149-158 (2000).
- Shields, S. D., Eckert, W. A., Basbaum, A. I. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic. 4, 465-470 (2003).
- Richner, M., Bjerrum, O. J., Nykjaer, A., Vaegter, C. B. The spared nerve injury (SNI) model of induced mechanical allodynia in mice. J. Vis. Exp. (54), (2011).
- Benbouzid, M., et al. Chronic, but not acute, tricyclic antidepressant treatment alleviates neuropathic allodynia after sciatic nerve cuffing in mice. Eur. J. Pain. 12, 1008-1017 (2008).
- Yalcin, I., et al. Β2-adrenoceptors are essential for desipramine, venlafaxine or reboxetine action in neuropathic pain. Neurobiol. Dis. 33, 386-394 (2009).
- Yalcin, I., et al. Β2-adrenoceptors are critical for antidepressant treatment of neuropathic pain. Ann. Neurol. 65, 218-225 (2009).
- Balasubramanyan, S., Stemkowski, P. L., Stebbing, M. J., Smith, P. A. Sciatic chronic constriction injury produces cell-type-specific changes in the electrophysiological properties of rat substantia gelatinosa neurons. J. Neurophysiol. 96, 579-590 (2006).
- Ikeda, T., et al. Effects of intrathecal administration of newer antidepressants on mechanical allodynia in rat models of neuropathic pain. Neurosci. Res. 63, 42-46 (2009).
- Thakor, D. K., et al. Increased peripheral nerve excitability and local NaV1.8 mRNA up-regulation in painful neuropathy. Mol. Pain. 5, 14 (2009).
- Zhu, Y. F., Wu, Q., Henry, J. L. Changes in functional properties of A-type but not C-type sensory neurons in vivo in a rat model of peripheral neuropathy. J. Pain Res. 5, 175-192 (2012).
- Cheng, H. Y., et al. DREAM is a critical transcriptional repressor for pain modulation. Cell. 108, 31-43 (2002).
- Zhang, J., De Koninck, Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J. Neurochem. 97, 772-783 (2006).
- Beggs, S., Liu, X. J., Kwan, C., Salter, M. W. Peripheral nerve injury and TRPV1-expressing primary afferent C-fibers cause opening of the blood-brain. Mol. Pain. 6, 74 (2010).
- Vachon, P., Massé, R., Gibbs, B. F. Substance P and neurotensin are up-regulated in the lumbar spinal cord of animals with neuropathic. 68, 86-92 (2004).
- Aouad, M., Petit-Demoulière, N., Goumon, Y., Poisbeau, P. Etifoxine stimulates allopregnanolone synthesis in the spinal cord to produce analgesia in experimental mononeuropathy. Eur. J. Pain. 18, 258-268 (2014).
- Pitcher, G. M., Ritchie, J., Henry, J. L. Paw withdrawal threshold in the von Frey hair test is influenced by the surface on which the rat stands. J. Neuroci. Methods. 87, 185-193 (1999).
- Beaudry, F., Girard, C., Vachon, P. Early dexamethasone treatment after implantation of a sciatic-nerve cuff decreases the concentration of substance P in the lumbar spinal cord of rats with neuropathic. Can. J. Vet. Res. 71, 90-97 (2007).
- Pitcher, G. M., Henry, J. L. Cellular mechanisms of hyperalgesia and spontaneous pain in a spinalized rat model of peripheral neuropathy: changes in myelinated afferent inputs implicated. Eur. J. Neurosci. 12, 2006-2020 (2000).
- Pitcher, G. M., Henry, J. L. Nociceptive response to innocuous mechanical stimulation is mediated via myelinated afferents and NK-1 receptor activation in a rat model of neuropathic pain. Exp. Neurol. 186, 173-197 (2004).
- Pitcher, G. M., Henry, J. L. Governing role of primary afferent drive in increased excitation of spinal nociceptive neurons in a model of sciatic neuropathy. Exp. Neurol. 214, 219-228 (2008).
- Lu, V. S., et al. Brain-derived neurotrophic factor drives the changes in excitatory synaptic transmission in the rat superficial dorsal horn that follow sciatic nerve injury. J. Physiol. 587, 1013-1032 (2009).
- Ruangsri, S., Lin, A., Mulpuri, Y., Lee, K., Spigelman, I., Nishimura, I. Relationship of axonal voltage-gated sodium channel 1.8 (NaV1.8) mRNA accumulation to sciatic nerve injury-induced painful neuropathy in rats. J. Biol. Chem. 286, 39836-39847 (2011).
- Zhu, Y. F., Henry, J. L. Excitability of AΒ sensory neurons is altered in an animal model of peripheral neuropathy. BMC Neurosci. 13, 15 (2012).
- Coull, J. A., et al. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 424, 938-942 (2003).
- Coull, J. A., et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 438, 1017-1021 (2005).
- Keller, A. F., Beggs, S., Salter, M. W., De Koninck, Y. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic. 3, 27 (2007).
- Fundytus, M. E., Fisher, K., Dray, A., Henry, J. L., Coderre, T. J. In vivo antinociceptive activity of anti-rat mGluR1 and mGluR5 antibodies in rats. Neuroreport. 9, 731-735 (1998).
- Fundytus, M. E., et al. Knockdown of spinal metabotropic glutamate receptor 1 (mGluR(1)) alleviates pain and restores opioid efficacy after nerve injury in rats. Br. J. Pharmacol. 132 (1), 354-367 (2001).
- Coderre, T. J., Kumar, N., Lefebvre, C. D., Yu, J. S. Evidence that gabapentin reduces neuropathic pain by inhibiting the spinal release of glutamate. J. Neurochem. 94, 1131-1139 (2005).
- Kabli, N., Cahill, C. M. Anti-allodynic effects of peripheral delta opioid receptors in neuropathic pain. Pain. 127, 84-93 (2007).
- Holdridge, S. V., Cahill, C. M. Spinal administration of a delta opioid receptor agonist attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Eur. J. Pain. 11, 685-693 (2007).
- Benbouzid, M., et al. Δ-opioid receptors are critical for tricyclic antidepressant treatment of neuropathic allodynia. Biol. Psychiatry. 63, 633-636 (2008).
- Bohren, Y., et al. µ-opioid receptors are not necessary for nortriptyline treatment of neuropathic allodynia. Eur. J. Pain. 14, 700-704 (2010).
- Yalcin, I., et al. Nociceptive thresholds are controlled through spinal Β2-subunit-containing nicotinic acetylcholine receptors. Pain. 152, 2131-2137 (2011).
- Bohren, Y., et al. Antidepressants suppress neuropathic pain by a peripheral Β2-adrenoceptor mediated anti-TNFα mechanism. Neurobiol. Dis. 60, 39-50 (2013).
- Choucair-Jaafar, N., Yalcin, I., Rodeau, J. L., Waltisperger, E., Freund-Mercier, M. J., Barrot, M. Β2-adrenoceptor agonists alleviate neuropathic allodynia in mice after chronic treatment. Br. J. Pharmacol. 158, 1683-1694 (2009).
- Yalcin, I., et al. Chronic treatment with agonists of Β2-adrenergic receptors in neuropathic pain. Exp. Neurol. 221, 115-121 (2010).
- Cok, O. Y., Eker, H. E., Yalcin, I., Barrot, M., Aribogan, A. Is there a place for Β-mimetics in clinical management of neuropathic pain? Salbutamol therapy in six cases. Anesthesiology. 112, 1276-1279 (2010).
- Choucair-Jaafar, N., et al. Cardiovascular effects of chronic treatment with a Β2-adrenoceptor agonist relieving neuropathic pain in mice. Neuropharmacology. 61, 51-60 (2011).
- Yalcin, I., et al. A time-dependent history of mood disorders in a murine model of neuropathic pain. Biol. Psychiatry. 70, 946-953 (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved