A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Percutaneous Hepatic Perfusion (PHP) with Melphalan as a Treatment for Unresectable Metastases Confined to the Liver
In This Article
Summary
In this manuscript, we describe percutaneous isolated hepatic perfusion with simultaneous chemofiltration as treatment for unresectable liver metastases. This procedure is performed under general anaesthesia in the angiosuite by an experienced team, consisting of an interventional radiologist, a clinical perfusionist and anaesthesiologist.
Abstract
Unresectable liver metastases of colorectal cancer can be treated with systemic chemotherapy, aiming to limit the disease, extend survival or turn unresectable metastases into resectable ones. Some patients however, suffer from side effects or progression under systemic treatment. For patients with metastasized uveal melanoma there are no standard systemic therapy options. For patients without extrahepatic disease, isolated liver perfusion (IHP) may enable local disease control with limited systemic side effects. Previously, this was performed during open surgery with satisfying results, but morbidity and mortality related to the open procedure, prohibited a widespread application. Therefore, percutaneous hepatic perfusion (PHP) with simultaneous chemofiltration was developed. Besides decreasing morbidity and mortality, this procedure can be repeated, hopefully leading to a higher response rate and improved survival (by local control of disease). During PHP, catheters are placed in the proper hepatic artery, to infuse the chemotherapeutic agent, and in the inferior caval vein to aspirate the chemosaturated blood returning through the hepatic veins. The caval vein catheter is a double balloon catheter that prohibits leakage into the systemic circulation. The blood returning from the hepatic veins is aspirated through the catheter fenestrations and then perfused through an extra-corporeal filtration system. After filtration, the blood is returned to the patient by a third catheter in the right internal jugular vein. During PHP a high dose of melphalan is infused into the liver, which is toxic and would lead to life threatening complications when administered systemically. Because of the significant hemodynamic instability resulting from the combination of caval vein occlusion and chemofiltration, hemodynamic monitoring and hemodynamic support is of paramount importance during this complex procedure.
Introduction
Resection of malignant liver tumors is the first choice of treatment for both primary and secondary hepatic malignancies. However, a large proportion of patients are not candidates for surgery because of extended disease or location of the metastases. For patients with unresectable metastases from colorectal carcinoma, systemic therapy is often the preferred treatment. Hepatic metastases from uveal melanoma are often small and diffusely spread throughout the liver. No standard systemic therapy is available for this group of patients. Local therapy can be an alternative to systemic treatment, in case the metastases are confined to the liver.
Because of the specific vascular anatomy of the liver, this organ can be isolated from the systemic circulation. This allows perfusion of the liver with high dose chemotherapy (IHP, isolated hepatic perfusion). Besides, liver malignancies have a dominant or exclusive vascular supply from the hepatic artery, whereas 70-80% of the supply of the non-tumorous liver parenchyma is derived from the portal vein.1,2 This technique was developed over twenty years ago, to treat patients with unresectable metastases from various primary origins.3,4 Especially, uveal melanoma patients with metastases in the liver may be candidates for IHP because the metastases are often small and spread throughout the entire liver, and at present no standard systemic therapy is available.5,6
The principle of IHP is to temporarily isolate the liver from the systemic circulation and perfuse the organ with a high dose of chemotherapy, leading to high local drug exposure with limited systemic side effects.7 This high dose of chemotherapy would be toxic and lead to complications when administered systemically. The majority of IHP studies were performed with melphalan, and have investigated treatment of hepatic metastasis from colorectal cancer patients, as well as patients with uveal melanoma metastases.8,9 Several studies of IHP during open surgery suggest that this treatment might be effective: 50-59% tumor response rates (partial and complete response) for the treatment of colorectal cancer and a 68% tumor response rate for patients with metastatic uveal melanoma have been reported.8,10,11,12 Despite these treatment results, this procedure never gained wide acceptance, because of the complexity of the procedure, the duration of hospital stay and the associated morbidity and mortality.
Percutaneous hepatic perfusion (PHP) offers a minimal invasive alternative to IHP and was first demonstrated in a porcine model in 1993 using doxorubicin13 and the first in human trial was performed by Ravikumar et al. in 1994.14 Due to lack of evidence of efficacy, the technique was largely abandoned until the early 2000's when it was re-evaluated in the National Cancer Institute (NCI) in the United States.15 During PHP, a catheter is placed percutaneous into the proper hepatic artery via the femoral artery to infuse the chemotherapeutic agent. A second catheter is placed in the inferior caval vein via the femoral vein to aspirate the hepatic chemosaturated outflow (see the PHP circuit in Figure 1). The isolation aspiration catheter placed in the caval vein is a double balloon catheter, prohibiting leakage into the systemic circulation. The aspirated chemosaturated blood is filtered by a double charcoal filter and returned to the patient by a third catheter placed in the internal jugular vein. The patient is admitted in the hospital with a length of stay of ~3 days. The PHP procedure is performed in an angiography room under general anaesthesia by a well-trained multidisciplinary team consisting of a dedicated interventional radiologist, anaesthesiologist and an extracorporeal perfusionist. A surgical oncologist and medical oncologist are also members of this multidisciplinary team, and especially focus on informing the patient, patient selection and post-operative care.
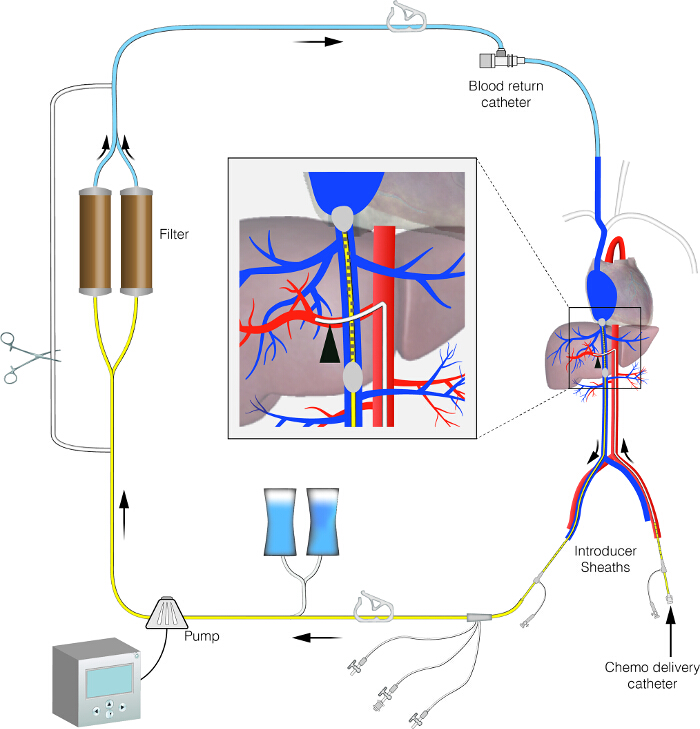
Figure 1. Schematic image of the PHP circuit. This figure displays the set-up of the PHP circuit. It shows an isolated hepatic perfusion circuit with extra-corporeal bypass line. Please click here to view a larger version of this figure.
This minimal invasive procedure is associated with less operative morbidity and can be repeated several times (at least up to 4 times). Besides, it only takes approximately 3 to 4 hr and patient recovery is fast. The advantage of PHP is the fact that all sizes of metastases can be treated, and micro metastases are being treated as well. Also the location of the metastases, close to vascular structures and bile ducts, is not a contraindication for PHP. Initial studies were performed with the 1st generation filter, with a 77% (mean) filter extraction efficiency.16 Recently, the results of a phase III trial were published by Hughes et al. showing a significant improvement of hepatic progression free survival in uveal melanoma patients with hepatic metastases treated with PHP compared to best alternative care.17
Since April 2012 a 2nd generation filter is available. In pre-clinical studies the 2nd generation filter is extracting 98% of melphalan. Several studies and case series investigating PHP for multiple indications have been published, but apart from the recent publishing phase III trial, survival has not extensively been analysed.16,18,19,20 In the present investigation, we focus on the interventional radiology procedure, as well as the anaesthetic management and the extra corporeal circulation that is used during this procedure in order to facilitate the use of this treatment in other medical centers.
Protocol
Note: After a patient met all inclusion criteria and was carefully evaluated by a medical oncologist, surgeon and anaesthesiologist, a patient was included in the study. All patients provided written informed consent. Both clinical studies were approved by the Local Medical Ethics Committee of the Leiden University Medical Centre and are performed in accordance with the ethical standards of the Helsinki Declaration.
1. Pre-procedural Angiography by Interventional Radiologist
- In preparation for the PHP procedure, perform a pre-procedural angiography20 several days prior to PHP for arterial anatomy mapping. During this procedure perform angiography of the superior mesenteric artery (SMA), coeliac trunk and hepatic arteries.21
- During the angiography, use contrast agent using a pump injector into the common hepatic artery and lobar arteries using a 2.4F or 2.7F microcatheter. (Material list, item 7).
- Inject 15 ml of non-diluted contrast (iopromide 300) (Material List, item 14) at a rate of 5ml/sec for angiography from the common hepatic artery and 12 ml at 3ml/sec for lobar injections.21
- Delineate the hepatic vascular anatomy and the vascular tumor supply by injecting contrast as described in step 1.1.2.21
- Determine the risk of reflux of the chemotherapeutic agent to hepatico-enteric anastomosis (such as the gastroduodenal or right gastric artery).21 Identify vascular variants, such as an aberrant right or left hepatic artery.21
- In case of a possible risk of reflux, if there is less than ~1 cm of proper hepatic artery distal to the gastroduodenal artery (GDA), embolize this artery with detachable microcoils or vascular plug (Material List, item 15 and 16). Also embolize other gastro- and/or enteric anastomoses to prevent flow of chemotheraputic drugs to other structures.21
- Embolize arterial branches distal of the site of infusion with vascular supply to the stomach, pancreas and intestines of diaphragm.21
2. PHP Procedure I: Preparation and Creating a Perfusion Circuit
- Preparation by Extra Corporeal Perfusionist (ECP)
- Collect the chemosaturation tubingpack, chemofilters and the centrifugal pump. (Material List, items 1b and 12) These items are packed in the box provided by the manufacturer.
- Flush the complete extracorporeal circuit with CO2 gas.
- Prime the circuit with NaCl 0.9% and heparin (2,000 IU/L) including the filters.
- Rinse the extracorporeal system with 6 L of heparinized 0.9% NaCl, initially by means of the force of gravity as a primeline is already present in the system. After the carbon filters are fully hydrated, use the centrifugal pump to put more pressure on the filters, in order to remove the remaining gas bubbles.
Note: A little hammer can be used to remove gas bubbles by vibration and resonance.- Prime double balloon catheter line and bypass line.
- Prime left chemofilter (with 1 L) and de-bubble, then prime right chemofilter (with 1 L) and de-bubble.
- Hydrate chemofilters (3 L per cartridge) after initial priming of the system slowly. This can take 10-20 min per chemofilter.
- Prime bubble trap (arterial filter).
- Rinse the extracorporeal system with 6 L of heparinized 0.9% NaCl, initially by means of the force of gravity as a primeline is already present in the system. After the carbon filters are fully hydrated, use the centrifugal pump to put more pressure on the filters, in order to remove the remaining gas bubbles.
- Check and calibrate the flow sensor and pressure monitors, as programmed in the centrifugal pump software menu, according to manufacturer's instructions.
- Patient Preparation on Angiography Room by Anaesthesiologist
- Set up the required monitoring for anaesthetic management:
- Place a blood pressure band for non-invasive blood pressure measuring ECG electrodes on patient's chest for ECG monitoring as well as SaO2 monitoring.
- Place a Bispectral Index Scale (BIS) sensor on patient's forehead to measure the patient's depth of anaesthesia, in this case the anesthesiologist is used to working with this.
- Set up ventilation equipment according to manufacturer's instructions, including ET-CO2 via the ventilation machine and endotracheal tube. Place the ventilation machine on the opposite side of the patient of where the IR is standing, as instructed by the manufacturer.
- Place the patient on angiography room table and directly cover the patient with a warm air blanket to mantain normothermia.
- Place a peripheral venous access in a right arm vein for administration of i.v. anaesthetics and i.v. fluids.
- Induce anaesthesia (with anaesthetics at the anaesthesiologist's preference), perform endotracheal intubation and start intermittent positive pressure ventilation (IPPV).
- Maintain anaesthesia to a BIS = 50, MAP = 65 mmHg, normuria of 0.5 ml/kg/hr.
- Place a nasopharyngeal body temperature thermometer.
- Place Train of four (TOF) electrodes for neuromuscular blockade monitoring as described in manufacturer's instructions (at the anesthesiologist preference).
- Set up and prepare a rapid fluid management system/or fluid warmer (Material List, item 2) as described in manufacturer's instructions.
- Turn on the advanced monitor screen (Material List, item 4) and connect all cables.
- Insert a 22 G arterial line into the left radial artery for mean arterial pressure (MAP) monitoring. (Material List, item 3).
- Place a central venous line for central venous pressure measurement in the left internal jugular vein.
- Insert a urinary bladder catheter.
- Maintain normothermia by warm air blankets and warm intravenous fluids.
- Monitor CVP and intra-arterial blood pressure using an advanced monitor (Material List, item 4) throughout the procedure.
- Determine a baseline activated clotting time (ACT) by drawing a blood sample and placing it in the ACT meter.
- In addition, at induction administer intravenous cefazolin 1 g, pantoprazol 40 mg and hydrocortisone 300 mg.
- Set up the required monitoring for anaesthetic management:
- Preparation by the Pharmacist
- Reconstitute melphalan (3.0 mg/kg body weight (maximal dose 220 mg) (Material List, item 9)) by rapidly injecting 10 ml of the supplied diluent directly into the vial of lyophilized powder using a sterile needle (20 G or larger needle diameter) and syringe.
- Immediately shake vial vigorously until a clear solution is obtained. This provides a 5 mg/ml solution of melphalan.
- Immediately dilute the dose to be administered in NaCl 0.9% injection, USP, to a concentration not greater than 0.45 mg/ml.
- Complete administration within 60 min of reconstitution.
- Preparation on Angiography Room by Interventional Radiologist (IR)
- Lay out the necessary tools and supplies (sterile) at a table. (Materials List, items 1a,3,5,6,7,8,11).
- Cover the patient with sterile sheets.
- Placement of the Sheaths by IR
- Use ultrasound guidance for the placement of sheaths in the internal jugular veins (IJV) both left and right.
- Place a 7.5F triple lumen intravenous catheter, 20 cm (Material List, item 5) in the left IJV. This sheath is used by the anaesthesiologist for CVP measurement and infusion of sympathomimetic drugs.
- Place a 10F venous return catheter (Material List, Item 1a) in the right IJV. This sheath is used for the venous return of the filtered blood after extracorporeal chemofiltration.
- Place a 5F sheath into the left common femoral artery (CFA) (Material List, Item 6).
Note: This sheath is used for the coaxially placed microcatheter to infuse the chemotherapeutic agent. - Place an 18F sheath into the right common femoral vein (CFV) (Material List, Item 8) after serial dilation with 8-10F and 12-14F dilators. Use the dilators to adjust the insertion opening to the right size to eventually properly place the 18F sheath. Note: This sheath is used to introduce the double balloon catheter (Isolation Aspiration Catheter).
- Isolation of the Hepatic Vein and Creating a Circuit Outside the Body
- Administer heparin after all sheaths have been placed; at an initial dose of 300 IU/kg (Material List, item 11).
- Maintain the activated clotting time (ACT) above 50 sec during the entire procedure with additional heparin bolus doses as required.
- Start norepinephrine (50 ml 0.2mg/ml) 0.1-2 µg/kg/min and phenylephrine (50 ml 0.1mg/ml) 0.1-2 µg/kg/min and fluid administration (500 ml of crystalloids) to maintain hemodynamic parameters close to a mean arterial pressure (MAP) of 65 mmHg, and urine output of 0.5 ml/kg/hr.
- Through the 5F sheath in the left common femoral artery, introduce the 2.7F microcatheter (Material List, Item 7) co-axially and place it in the proper hepatic artery or lobar arteries using angiography monitoring.
- Through the 18F sheath in the right common femoral vein, introduce the 16F double balloon catheter (Isolation Aspiration Catheter) and place it with the tip in the right atrium using angiography monitoring.
- Directly after the placement of the catheters, attach these to the extracorporeal system using the special connection points; the venous return line to the sheath in the IJV and the outflow lines to the isolation catheter.
- Start the centrifugal pump as described by the manufacturer:
Note: All lines are open now and flow is running over bypass line. - After intravenous infusion of 0.5 L colloid solution (Material List, Item 13), inflate the cranial balloon with a 50% saline and 50% contrast media mixture.
- With the caudal balloon still deflated, slowly retract the double balloon catheter until the cranial balloon is at the junction of the right atrium and the inferior caval vein (IVC), just above the right hepatic vein (the balloon is now wedge-shaped, as illustrated in Figure 2).
- Inflate the caudal balloon with a mixture of 90% saline and 10% contrast media until lateral edges of the inflated balloon start to become effaced by the IVC.
Note: Balloons are inflated with different mixtures of contrast media. The caudal balloon is inflated to obstruct the inferior vena cava above the renal veins, so that hepatic venous outflow is isolated from systemic venous circulation. A volume of maximal 38 ml may be injected into each balloon. - Check positioning of the balloons and rule out leakage with venography
- Perform venography by injecting 20 ml of contrast (i.e., iopromide 300) through the fenestration port of the double balloon venous double balloon catheter using a hand injection.
- If contrast leakage is seen, reposition the balloons or further inflate the balloons and repeat the venography as described in step 2.6.11.1 (See Figure 2).
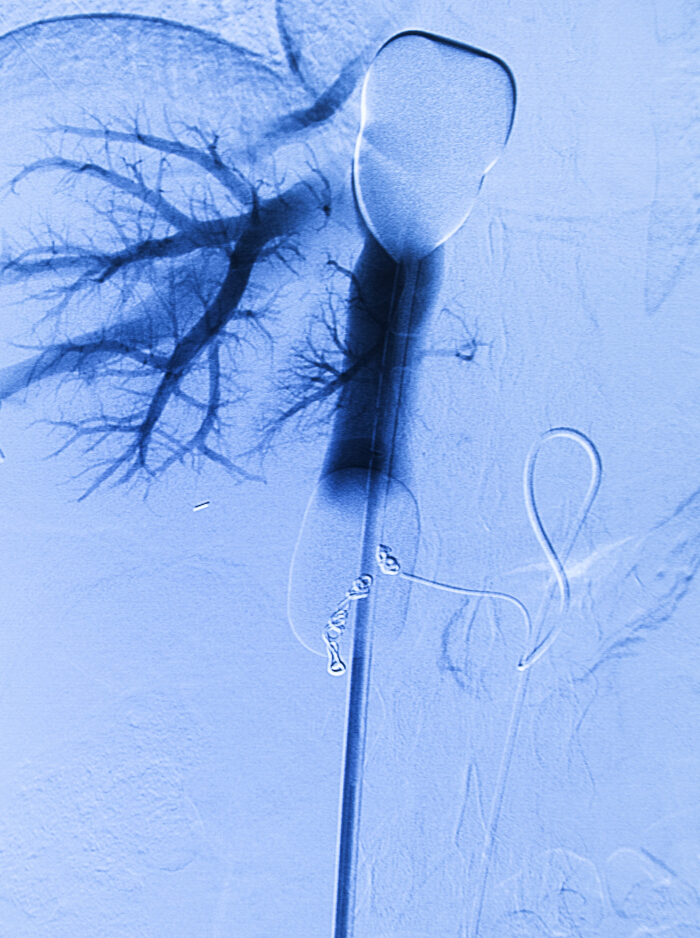
Figure 2. Per-procedural angiogram. Venous double balloon catheter in the inferior caval vein and arterial infusion catheter in the proper hepatic artery. Retrograde contrast is injected via the venous catheter. Coils from the pre-procedural angiography and embolization are in place. Please click here to view a larger version of this figure.
- Closing the Circuit
- Infuse another 0.5 L of colloid solution. Ensure that the two chemofilters are closed at this moment in time, while the bypass is open.
- Try to accomplish sufficient flow (Q = 400-500 ml/min) with safe pressures proximal (in general <-70 mmHg) and distal (<100 mmHg) of the centrifugal pump (Material List, item 12).
- Titrate norepinephrine 0.1-2 µg/kg/min and phenylephrine 0.1-2 µg/kg/min and fluid administration to maintain a systolic BP of 160 mmHg, and urine output of 0.5 ml/kg/hr.
- When sufficient flows and pressures are reached, open the filters one by one.
Note: Passage of blood though the filters will hemofiltrate the blood and remove the majority of the chemotherapeutic agent. Hemofiltration occurs throughout the procedure as well as in the 'washout' period directly following the chemotherapy-infusion period. During this washout period, chemotherapeutic agent that returns from the liver tissue, is 'washed out' from the circulation. - When a systolic BP between 140-160 mmHg has been reached, close the bypass line.
Note: The anaesthesiologist should be prepared to encounter a drop in blood pressure resulting from the reduction in preload due to caval vein occlusion, peripheral vasodilation from passage of blood through the chemofilters removal of vasoactive agents (e.g., norepinephrine and phenylephrine) by the chemofilters and possibly the vasodilative effects of endogenous NO production. Adjust infusion rates of norepinephrine and phenylephrine accordingly maintaining a MAP of 65 mmHg. - Check positioning of the double balloon catheter by performing a venography (as described in step 2.6.11.1).
- Perform an angiogram by injecting contrast (iopromide 300) through the microcatheter in the hepatic artery to rule out arterial spasm. If spasm is seen, administer 100-200 micrograms of nitroglycerine into the hepatic artery (as also described in step 1.1 and sub-steps).
3. PHP Procedure II: Infusion of Chemotherapy and Washout Period
- Infusion of Melphalan
Note: After the placement of the delivery system circuit, infusion of melphalan into the hepatic artery is started through the microcatheter.- When a steady flow rate is established, no leakage is present and the patient's blood pressure is stable, inject melphalan at a dose of 3 mg/kg into the proper hepatic artery or sequentially into both lobar arteries.
- Do not start chemotherapy infusion until the MAP is above 65 mmHg.
- Inject the drug in doses of 100 ml using an auto-injector at a rate of 0.4 cc/sec, for approximately 30 min, until the complete dose is injected.
- During infusion, at regular intervals, perform an arteriogram to monitor the occurrence of vasospasm and the position and check inflation of the double balloon catheter with contrast flushing. (See step 2.6.11.1 (venography) and 1.1 and 2.7.9 (angiography) for information on the contrast agent).
- In case of vasospasm, administer nitro-glycerine (100-200 µg).
- After the 30 min of chemotherapeutics infusion, continue hemofiltration for an additional 30 min washout period (See Note at step 2.7.5).
- Administer 10 mg of i.v. morphine for postoperative pain control.
- After the washout period slowly deflate the two balloons in the caval vein.
- When a steady flow rate is established, no leakage is present and the patient's blood pressure is stable, inject melphalan at a dose of 3 mg/kg into the proper hepatic artery or sequentially into both lobar arteries.
- End of the Infusion and Removal of the Catheters
- At the end of the washout period, empty the extracorporeal circuit as much as possible and then stop the extracorporeal perfusion.
- Stop the centrifugal pump and disconnect the tubes.
- Remove the double balloon catheter and the infusion microcatheter, but leave the access sheaths in place until the coagulation is normalized. A vascular closure device may be placed in the common femoral artery to achieve haemostasis.
- Keep the return catheter in the right IJV in place until coagulation profile is normalized.
- Keep the 7.5F triple lumen intravenous catheter in the left IJV in place for this can be used at the Post Anaesthesia Care Unit (PACU).
- End of Anaesthesia and Heparinization
Note: Perform the following steps at the end of the procedure, when the auto-transfusion is finished.- Close and remove the bypass circuit.
- Stop the infusion of norepinephrine and phenylephrine immediately after double balloon deflation. This is possible due to the return of sufficient preload to the right heart and the termination of the chemofiltration.
- Normalize coagulation with protamine (1:1), fresh frozen plasma and/or cryoprecipitate with or without transfusion of thrombocytes. Check ACT afterwards.
- After hemodynamic stabilization and termination of norepinephrine and phenylephrine infusion, terminate the anesthetic maintenance medication.
- After the patient has returned to spontaneous ventilation and consciousness, extubate the trachea of the patient.
- Transfer the patient to the PACU for 24 hr of monitoring of vital functions (blood pressure and coagulation parameters).
- Remove the vascular sheath once the platelet count >50 x 109/L, APTT <1.5 x normal, and the INR <1.5. Apply manual compression to achieve haemostasis and apply a pressure bandage over both groins.
- Postoperative Care
- Monitor patients at the PACU for the first 24 hr after the procedure.
- Check hemostasis site of catheter insertion and monitor pulse, blood pressure, temperature, ventilation, fluid balance and pain scores as well as coagulation.
- Transfer the patient to the postoperative ward for 1-2 days.
- Discharge the patient at postoperative day 2 of 3, after a final haematology and chemistry blood test. Check for hepatotoxicity and create a baseline for follow-up on toxicity - prothrombin time (PT) within 2 sec of upper limit of normal, Activated Partial Thromboplastin Time(APTT) within normal range, platelets >75,000/mm3 without platelet transfusion or >100,000/mm3 with transfusion, haemoglobin >10 g/dl (>6.2 mmol/L) and aspartate aminotransferase and alanine aminotransferase (AST/ALT) returned to within 10% of baseline levels.
- Administer colony stimulating growth factor support (pegfilgastrim, 0.6 ml disposable syringe) upon discharge or within 48 hr after the PHP treatment.
Results
Knowledge about PHP is based on small phase I and II trials and case series and a recent larger phase trial III; an overview of published results is shown in Table 1. One paper discusses the anaesthesiology procedure, hemodynamic and metabolic aspects of the treatment. However, no survival data were reported.22 Three larger trials that were reported, included metastatic liver disease from different primary tumors and the results are therefore difficult to inter...
Discussion
Patients with unresectable liver metastases can be treated with systemic therapy. However, for patients with metastatic uveal melanoma, no standard systemic therapy is available and immunotherapy or targeted therapy have not yet been able to show improved survival. Isolated hepatic perfusion has been shown to be an effective treatment for patients with unresectable uveal melanoma metastases confined to the liver.9,28 For colorectal cancer metastases more therapeutic systemic options are available, but some pat...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors have no acknowledgements.
The phase II study is financially supported by Delcath Systems.
Materials
| Name | Company | Catalog Number | Comments |
| Delcath 2nd Generation Hepatic CHEMOSAT Delivery System | Delcath Systems Inc., New York, New York, USA | 602001 and 602002 | Item no. 1 |
| Isofuse Isolation Aspiration Catheter (Double Balloon Catheter) | Delcath Systems Inc., New York, New York, USA | Item no. 1a | |
| 10F Venous Return Catheter | Delcath Systems Inc., New York, New York, USA | ||
| Hemofiltration Cartridges | Delcath Systems Inc., New York, New York, USA | Item no. 1b | |
| Circuit components | Delcath Systems Inc., New York, New York, USA | ||
| Level-1 rapid fluid management system | Smiths Medical | Item no. 2 | |
| 22 G arterial line | Arrow International Inc. / Teleflex Inc.,Dublin, Ireland | Item no. 3 | |
| Vigileo, Monitor | Edwards Lifesciences Corp, Irvine, California, USA | Item no. 4 | |
| 7.5F Triple lumen intravenous catheter, 20 cm | Vygon, Valkenswaard, Nederland | Item no. 5 | |
| 5F sheath | Item no. 6 | ||
| 2.7 Progreat microcatheter | Terumo, Tokyo, Japan | MC-PP27131 | Item no. 7 |
| 18F sheath | Item no. 8 | ||
| Auto-injector Medrad Mark V ProVis | Bayer, Indianola, Pennsylvania, USA | Not available anymore, replaced by Medrad Mark 7 Arterion Injection System | Item no. 9 |
| Melphalan | Alkeran, Aspen Pharmacare, Dublin, Ireland | Item no. 10 | |
| Heparin LEO, 5,000 IE/ml | LEO Pharma AB, Denmark | Item no. 11 | |
| Centrifugal pump | Medtronic, Minneapolis, Minnesota, VS | Item no. 12 | |
| Voluven colloid solution (6% hydroxyethyl starch 130/0.4 in 0.9% sodium chloride injection) | Item no. 13 | ||
| Iopromide 300, Ultravist | Bayer, Indianola, Pennsylvania, USA | Item no. 14 | |
| Detachable coil, Interlock | Boston Scientific, Marlborough, Massachusetts, USA | Item no. 15 | |
| Vascular plug, Amplatzer 4 | St. Jude Medical, St Paul,Minnesota, USA | Item no. 16 |
References
- Jovanovic, P., et al. Ocular melanoma: an overview of the current status. Int J Clin Exp Pathol. 6 (7), 1230-1244 (2013).
- Taylor, I., Bennett, R., Sherriff, S. The blood supply of colorectal liver metastases. Br J Cancer. 38 (6), 749-756 (1978).
- van de Velde, C. J., et al. A successful technique of in vivo isolated liver perfusion in pigs. J Surg Res. 41 (6), 593-599 (1986).
- Vahrmeijer, A. L., Der Eb, M. M. V. a. n., Van Dierendonck, J. H., Kuppen, P. J., Van De Velde, C. J. Delivery of anticancer drugs via isolated hepatic perfusion: a promising strategy in the treatment of irresectable liver metastases?. Semin Surg Oncol. 14 (3), 262-268 (1998).
- Vahrmeijer, A. L., van de Velde, C. J., Hartgrink, H. H., Tollenaar, R. A. Treatment of melanoma metastases confined to the liver and future perspectives. Dig Surg. 25 (6), 467-472 (2008).
- Ben-Shabat, I., et al. Isolated hepatic perfusion as a treatment for liver metastases of uveal melanoma. J Vis Exp. (95), e52490 (2015).
- Vahrmeijer, A. L., et al. Increased local cytostatic drug exposure by isolated hepatic perfusion: a phase I clinical and pharmacologic evaluation of treatment with high dose melphalan in patients with colorectal cancer confined to the liver. Br J Cancer. 82 (9), 1539-1546 (2000).
- Rothbarth, J., et al. Isolated hepatic perfusion with high-dose melphalan for the treatment of colorectal metastasis confined to the liver. Br J Surg. 90 (11), 1391-1397 (2003).
- van Iersel, L. B., et al. Isolated hepatic perfusion with 200 mg melphalan for advanced noncolorectal liver metastases. Ann Surg Oncol. 15 (7), 1891-1898 (2008).
- van Iersel, L. B., et al. Isolated hepatic melphalan perfusion of colorectal liver metastases: outcome and prognostic factors in 154 patients. Ann Oncol. 19 (6), 1127-1134 (2008).
- Alexander, H. R., et al. A phase I-II study of isolated hepatic perfusion using melphalan with or without tumor necrosis factor for patients with ocular melanoma metastatic to liver. Clin Cancer Res. 6 (8), 3062-3070 (2000).
- Olofsson, R., et al. Isolated hepatic perfusion for ocular melanoma metastasis: registry data suggests a survival benefit. Ann Surg Oncol. 21 (2), 466-472 (2014).
- Curley, S. A., et al. Increased doxorubicin levels in hepatic tumors with reduced systemic drug exposure achieved with complete hepatic venous isolation and extracorporeal chemofiltration. Cancer Chemother Pharmacol. 33 (3), 251-257 (1993).
- Ravikumar, T. S., et al. Percutaneous hepatic vein isolation and high-dose hepatic arterial infusion chemotherapy for unresectable liver tumors. J Clin Oncol. 12 (12), 2723-2736 (1994).
- Lillemoe, H. A., Alexander, H. R. Current Status of Percutaneous Hepatic Perfusion as Regional Treatment for Patients with Unresectable Hepatic Metastases: A Review. Am Oncology and Hematology Rev. (15-23), (2014).
- Pingpank, J. F., et al. Phase I study of hepatic arterial melphalan infusion and hepatic venous hemofiltration using percutaneously placed catheters in patients with unresectable hepatic malignancies. J Clin Oncol. 23 (15), 3465-3474 (2005).
- Fitzpatrick, M., et al. Use of partial venovenous cardiopulmonary bypass in percutaneous hepatic perfusion for patients with diffuse, isolated liver metastases: a case series. J Cardiothorac Vasc Anesth. 28 (3), 647-651 (2014).
- Forster, M. R., et al. Chemosaturation with percutaneous hepatic perfusion for unresectable metastatic melanoma or sarcoma to the liver: a single institution experience. J Surg Oncol. 109 (5), 434-439 (2014).
- Deneve, J. L., et al. Chemosaturation with percutaneous hepatic perfusion for unresectable isolated hepatic metastases from sarcoma. Cardiovasc Intervent Radiol. 35 (6), 1480-1487 (2012).
- Kandarpa, K., Machan, L. . Handbook of Interventional Radiologic Procedures. , 269-270 (2011).
- Hofmann, H., et al. Unresectable isolated hepatic metastases from solid pseudopapillary neoplasm of the pancreas: a case report of chemosaturation with high-dose melphalan. Pancreatology. 14 (6), 546-549 (2014).
- Miao, N., et al. Percutaneous hepatic perfusion in patients with metastatic liver cancer: anesthetic, hemodynamic, and metabolic considerations. Ann Surg Oncol. 15 (3), 815-823 (2008).
- Vogl, T. J., et al. Chemosaturation with percutaneous hepatic perfusions of melphalan for hepatic metastases: experience from two European centers. Rofo. 186 (10), 937-944 (2014).
- Pingpank, J. F., et al. A phase III random assignment trial comparing percutaneous hepatic perfusion with melphalan (PHP-mel) to standard of care for patients with hepatic metastases from metastatic ocular or cutaneous melanoma. J Clin Oncol. 28, 18s (2010).
- Rothbarth, J., Vahrmeijer, A. L., Mulder, G. J. Modulation of cytostatic efficacy of melphalan by glutathione: mechanisms and efficacy. Chem Biol Interact. 140 (2), 93-107 (2002).
- Bartlett, D. L., Libutti, S. K., Figg, W. D., Fraker, D. L., Alexander, H. R. Isolated hepatic perfusion for unresectable hepatic metastases from colorectal cancer. Surgery. 129 (2), 176-187 (2001).
- van Iersel, L. B., et al. Isolated hepatic perfusion with oxaliplatin combined with 100 mg melphalan in patients with metastases confined to the liver: A phase I study. Eur J Surg Oncol. , (2014).
- Rothbarth, J., Tollenaar, R. A., van de Velde, C. J. Recent trends and future perspectives in isolated hepatic perfusion in the treatment of liver tumors. Expert Rev Anticancer Ther. 6 (4), 553-565 (2006).
- van Etten, B., de Wilt, J. H., Brunstein, F., Eggermont, A. M., Verhoef, C. Isolated hypoxic hepatic perfusion with melphalan in patients with irresectable ocular melanoma metastases. Eur J Surg Oncol. 35 (5), 539-545 (2009).
- van Iersel, L. B., et al. Management of isolated nonresectable liver metastases in colorectal cancer patients: a case-control study of isolated hepatic perfusion with melphalan versus systemic chemotherapy. Ann Oncol. 21 (8), 1662-1667 (2010).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved