A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Methods for Acute and Subacute Murine Hindlimb Ischemia
In This Article
Summary
Surgical induction of hindlimb ischemia in the mouse is useful to examine angiogenesis, however this is compromised in certain inbred mouse strains that display marked ischemia-induced tissue necrosis. Methods are described to induce subacute limb ischemia using ameroid constrictors to circumvent this problem through the induction of gradual arterial occlusion.
Abstract
Peripheral artery disease (PAD) is a leading cause of cardiovascular morbidity and mortality in developed countries, and animal models that reliably reproduce the human disease are necessary to develop new therapies for this disease. The mouse hindlimb ischemia model has been widely used for this purpose, but the standard practice of inducing acute limb ischemia by ligation of the femoral artery can result in substantial tissue necrosis, compromising investigators' ability to study the vascular and skeletal muscle tissue responses to ischemia. An alternative approach to femoral artery ligation is the induction of gradual femoral artery occlusion through the use of ameroid constrictors. When placed around the femoral artery in the same or different locations as the sites of femoral artery ligation, these devices occlude the artery over 1 - 3 days, resulting in more gradual, subacute ischemia. This results in less substantial skeletal muscle tissue necrosis, which may more closely mimic the responses seen in human PAD. Because genetic background influences outcomes in both the acute and subacute ischemia models, consideration of the mouse strain being studied is important in choosing the best model. This paper describes the proper procedure and anatomical placement of ligatures or ameroid constrictors on the mouse femoral artery to induce subacute or acute hindlimb ischemia in the mouse.
Introduction
Peripheral artery disease (PAD) is a leading cause of cardiovascular morbidity and mortality in developed countries 1. PAD results from atherosclerotic obstruction of the peripheral arteries that leads to limb ischemia with resultant exertional or rest pain and occasionally non-healing ulcers and gangrene that necessitate limb amputation. Therapies targeting PAD are directed primarily towards endovascular 2 or surgical revascularization 3, as essentially no effective medical therapies exist 4.
Unfortunately, revascularization is often of limited benefit, as bypass grafts have high failure rates (up to 50% within 5 years) 5 that are worse in some populations (e.g., smokers, women, non-saphenous vein grafts) 6,7. Endovascular approaches, such as angioplasty and stenting, are also compromised by high restenosis rates (in excess of 50% within 1 year), particulary in femoropopliteal disease 8, although the use of drug-eluting balloons and stents has improved outcomes somewhat 9-11. In order to develop new treatments for PAD it is essential to develop animal models that reliably reproduce the human disease.
To date, the most common model of PAD is the hindlimb ischemia model (HLI), which is most frequently performed in mice 12,13. In its most common manifestation, the model entails surgical ligation of the proximal and distal femoral artery and its intervening side-branches followed by excision of the vessel, resulting in occlusion of blood flow and induction of acute limb ischemia. HLI has been used primarily to study the angiogenic and arteriogenic responses in peripheral limb muscle tissue and the effects of various therapies (e.g., drugs, gene delivery, stem cells) on these responses. More recently, our group has used this model to examine the role of skeletal muscle cells in the response to limb ischemia and the effects of genetic differences on outcomes 14.
The HLI model has facilitated our current understanding that the vascular and muscle responses to ischemia are dependent on genetics (i.e., inbred strain) 15, age 16, and the presence or absence of other diseases or conditions relevant to atherosclerosis, including diabetes mellitus 17 and hypercholesterolemia 18. However, an important weakness of the traditional HLI model is that it is a model of acute limb ischemia 12,13, whereas human PAD causes chronic ischemia as a result of the gradual development of occlusive atherosclerotic lesions in the peripheral arteries.
In an attempt to circumvent this weakness, Tang and colleagues initially developed a rat model of gradual femoral arterial occlusion using ameroid constrictors 19, and the same group subsequently developed a similar mouse model 20. Ameroid constrictors were described initially in the 1950s in a canine model of chronic myocardial ischemia 21,22. These devices have an outer metal sleeve encasing an inner layer of a hygroscopic material, usually casein, and when placed around an artery they induce gradual vessel occlusion as they absorb moisture from the surrounding tissues. In their modification of the model, Yang et al. placed constrictors on both the proximal and distal femoral artery at sites analogous to the surgical ligation sites, and they ligated the side branches of the femoral artery, as in the traditional model. Compared to acute HLI, ameroid constrictor-induced ischemia resulted in lower expression of inflammatory and shear stress-dependent genes, lower blood flow recovery 4 - 5 weeks post-operatively, and less muscle necrosis 20. Based on these observations, it was felt that gradual arterial occlusion might provide a model of PAD more relevant to the human disease.
Notably, in the original report, effects of ameroid constrictor-induced ischemia were examined only in C57BL/6 mice 19, which are relatively resistant to ischemia-induced muscle necrosis 15. We recently modified the gradual ischemia model further and explored its effects in the more ischemia-susceptible BALB/c mouse strain 23. In the first manifestation of the model, we placed constrictors on both the proximal and distal femoral artery but left all side-branches intact. In a second, milder modification, we placed a single constrictor only on the proximal femoral artery and again left all side-braches of the artery intact. In both modifications of this model, we found that BALB/c mice, but not C57BL/6 mice, displayed significant muscle necrosis despite having similar blood flow and vascular density. Similar to our previous study 14, these findings demonstrated that limb muscle injury is not solely influenced by blood flow, but is in part dependent on genetic background. Moreover, we found that limb blood flow fell to its nadir within 3 days, thus the model appears to be more one of 'subacute' rather than gradual limb ischemia.
Based on these prior studies, it appears clear that a single method for inducing hindlimb ischemia may not be suitable in all cases. Because a variety of conditions (e.g., genetic differences and presence or absence of co-morbid conditions) influence both the vascular and skeletal muscle-specific responses, investigators may find it necessary to modify the chronicity and/or the severity of hindlimb ischemia to best suit their purposes. Furthermore, prior descriptions of the model typically lacked suitable anatomical landmarks to facilitate reliable inter-investigator reproducibility of the technique. In this paper, methods for inducing either acute or subacute hindlimb ischemia in the mouse are described, and precise anatomical landmarks are provided.
Protocol
All animal experiments were performed according to protocol approved by the Duke Institutional Animal Care and Use Committee. Male mice were used in this study, although either sex can be used as indicated for the scientific purpose of the study.
1. Hair Removal
- Prior to induction of anesthesia, set up a pre-surgical preparation area consisting of a covered heating pad set at 37 °C and a nosecone port connected to continuous flow of isoflurane.
- Place the mouse in the anesthesia induction chamber. Set the O2 flow meter to 1 L/min and isoflurane to 1 - 3%.
NOTE: Anesthesia is typically induced in a 25 g mouse with 2% isoflurane. - Check the mouse's stimulus response by gently rocking the chamber and observing a lack of a righting reflex.
- Flush the chamber with O2 to clear the isoflurane prior to opening. Quickly move the mouse to the heating pad and connect it to isoflurane via the nosecone.
- Adjust the isoflurane to 1.5%. Check the stimulus response by pedal reflex (toe pinch).
- Apply ophthalmic lubricant liberally to both eyes to avoid drying during surgery.
- Shave the hair from both hind limbs using a small electric trimmer. Hold the skin taut while shaving to avoid lacerating the skin.
- Apply pre-warmed hair removal cream and let sit for 1 min. Gently wipe away using a moistened gauze pad.
- For surgical procedure at a later time, turn off the isoflurane and move the mouse to an empty paper towel-lined recovery cage to ensure the mouse does not aspirate the cage bedding. Monitor the animal until it is able to maintain sternal recumbency. Otherwise, move the mouse to the surgical table.
NOTE: The hair removal process can irritate the skin and affect perfusion measurements. It is recommended to wait 1 - 2 days after removing the animal's hair before performing a pre-surgical perfusion scan or performing surgery.
2. Pre-Surgical Preparation
- Use the following tools during this procedure; small straight surgical scissors, 2 fine-tipped angled forceps, small Graefe forceps, needle driver forceps, 3 retractors, small spring scissors, and fine-tipped cotton swabs.
- Sterilize all tools using an autoclave prior to the initiation of surgery. Use a hot-bead sterilizer before and between each surgical procedure, for up to 5 animals. Sterilize additional surgical tool packets for surgeries of groups larger than 5.
- Prepare a sterile surgical field consisting of a covered heating pad and an isoflurane port. Perform all work under a 10 stereo dissection microscope.
- Anesthetize and prepare the mouse as described in steps 1.1 to 1.5.
- Check that the mouse is fully sedated and place into a supine position on the surgical table. Secure both legs using surgical tape.
- If using a temperature-controlled heating pad, attach the temperature probe and secure it to the base of the surgical platform using surgical tape to ensure that it will not be accidentally pulled out during the procedure.
- Clean the incision site using 3 alternating povidone-iodine and alcohol wipes. Cover the animal with a sterile surgical drape and cut a hole to expose the incision site.
3. Induction of Limb Ischemia
- Use a scalpel to make an initial incision along the center of the medial thigh, running from the knee towards the abdomen, and lengthen the incision to approximately 1 cm with fine scissors (Figure 1A).
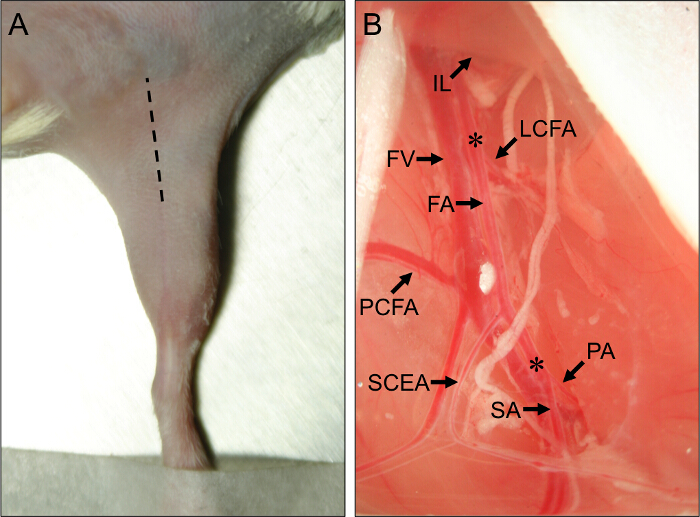
Figure 1. Surgical Site and Vascular Anatomical Landmarks for Mouse Hindlimb Ischemia Surgery. (A) External view of the hindlimb of a mouse in the supine position. The hatched line indicates the incision site to properly perform the hindlimb ischemia procedures. (B) View of the proximal mouse hindlimb vasculature. The proximal end of the femoral artery (FA) arises from beneath the inguinal ligament (IL). The distal end of the FA is located at its bifurcation into the popliteal artery (PA) and saphenous artery (SA). The major collateral arteries off of the FA are the lateral circumflex femoral artery (LCFA), the proximal caudal femoral artery (PCFA), and the superficial caudal epigastric artery (SCEA). The femoral vein (FV) runs adjacent to the FA, and venous branches can be seen parallel to the major arterial branches. Asterisks (*) denote the proximal and distal sites for placement of ameroid constrictors or ligatures, depending on whether subacute or acute ischemia will be induced. Please click here to view a larger version of this figure.
- Using forceps, open the incision and expose the membrane covering the inguinal fat tissue (IFT).
- Using closed-forceps, pierce through the membrane into the separation between the IFT and the abdomen. Gently release pressure on the forceps to separate the IFT from the abdominal muscles, exposing the neurovascular bundle underneath. Observe the proximal and superficial caudal branches as important anatomical landmarks (Figure 1B).
- Insert a retractor and pull the abdominal tissue proximally to expose the proximal ameroid constrictor or ligation site, just proximal to the lateral circumflex femoral artery (Figure 1B). The lateral circumflex artery lies about 5 mm proximal to the proximal and superficial caudal arteries.
- Insert two more retractors into the distal part of the incision, one medial and one lateral, to pull the IFT distally away from the surgical site to widen the surgical field.
- Use two fine forceps to remove the outermost membrane covering the neurovascular bundle. Gently insert one half of the fine forceps tip between the vein and artery, sliding the forceps tip under the membrane that binds them together. Close the forceps and gently tear away the membrane.
- Insert the tip of a closed forceps between the vein and artery and create a gap between them by releasing pressure on the forceps. Repeat this technique to create a gap between the artery and nerve.
- For subacute limb ischemia, place an ameroid constrictor on the proximal femoral artery (Figure 2).
- To install the proximal ameroid constrictor, slide the tip of a forceps under the femoral artery to isolate it from the neurovascular bundle. Use a second set of angled- forceps to grip the edge of the constrictor and guide it under the femoral artery.
- Lay the femoral artery into the slot in the constrictor. Repeat for the distal constrictor, positioning it immediately proximal to the bifurcation of the femoral artery into the popliteal artery and saphenous artery (Figure 2).
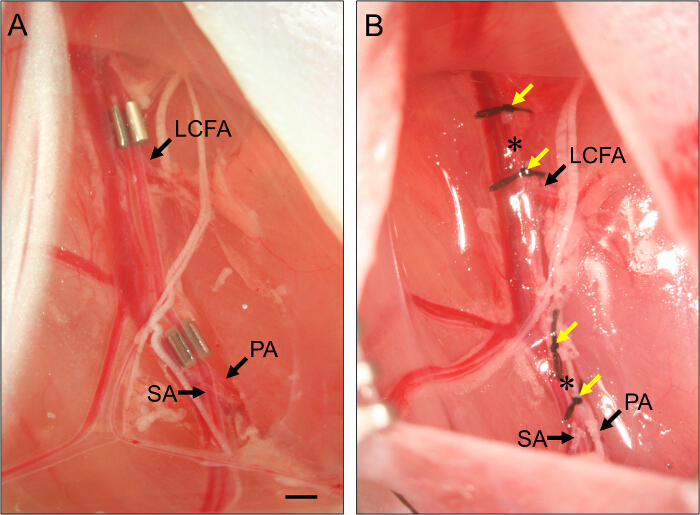
Figure 2. Placement of Ameroid Constrictors and Ligatures. (A) Example of two ameroid constrictors placed on the femoral artery to induce subacute hindlimb ischemia. The proximal constrictor is placed just proximal to the lateral circumflex femoral artery (LCFA). The distal constrictor is placed just proximal to the bifurcation of the popliteal (PA) and saphenous arteries (SA). Constrictors are installed with the slot facing up to ensure the artery is properly set within the constrictor. (B) Example of ligatures of the femoral artery to induce acute hindlimb ischemia. Ligatures (yellow arrows) are placed such that they flank the position of the constrictors in panel (B), and the femoral artery is transected between each set of two ligatures (asterisks). Bar, 1 mm. Please click here to view a larger version of this figure.
- For acute limb ischemia, ligate and transect the proximal femoral artery.
- To transect the femoral artery, thread 7 - O suture under the artery just proximal to the position of the proximal constrictor (see Step 3.7) and ligate. Tie a second ligature about 1 mm distal to the first.
- Use spring scissors to transect the artery between the two ligatures. For the distal arterial transection, repeat these steps, placing two ligatures about 1 mm apart, just proximal to the bifurcation of the femoral artery into the popliteal artery and saphenous artery but ensuring that they are distal to the superficial caudal epigastric artery (see Figure 1)
- Close the incision using interrupted 5 - O vicryl sutures.
4. Perfusion Imaging
- Move the mouse to a 37 °C heating pad set beneath the laser doppler perfusion imager (LDPI) and connect via a nosecone to the isoflurane source. If no temperature monitor is available, allow 5 min for the mouse to warm up to 37 °C.
- Turn on the imager and launch the image capture software.
- Click the 'New Single Image' icon to open the 'Scanner Setup' window. Set the 'Scan Size' to 'Large' and the 'Scan Speed' to '4 ms/pixel'. Set the scan area by changing the x and y values under the 'Scan Area (units)' pane.
- Click the 'Video and Distance' tab to view the video feed, and arrange the mouse to fit into the scan area indicated by a red outline. Click 'Auto Distance' to calibrate the distance from the laser to the subject. Click 'Next' to open the 'Subject Details' window.
- Enter the subject information and any relevant comments. Click the 'Next' button to move on to the scanning window.
- Click the 'Start Measurement' button to open the 'Confirm or Override Scan Distance' dialog. Click 'OK' to confirm scan distance. Observe the scanning process begin and run for 4 - 8 min depending on the size of the scan area.
- After the scanning is complete, observe the 'Save As' window. Name the file and save it.
- Shut off the isoflurane and move the mouse to an empty recovery cage and monitor until the animal is able to maintain sternal recumbency. Never place a mouse recovering from anesthesia into a cage with other mice.
- Open the image analysis software. Click the 'Open' icon and browse to and open the image file for analysis. On the file window, observe the flux, photo, and color images of the mouse.
- To mark the region of interest (ROI) on the flux image, click the 'Show ROIs' icon. Next click the 'Add Polygon' button and drag the cursor around the non-surgical limb to draw the ROI. Right-click to close the shape. Select 'Add Polygon' again and draw a matching ROI around the surgical limb.
- Click the 'Statistics' icon to open the 'Image ROIs Statistic Results (PU)' window. Observe the percent difference in flux in the 'Flux %' column.
NOTE: The first ROI drawn will serve as the reference.
NOTE: Prior to each subsequent perfusion scan follow the steps outlined in Section 1 to anesthetize the mouse and in steps 4.1 to 4.11 to image the animal.
Results
Proper identification of the mouse hindlimb vasculature is critical to ensuring reproducibility of the techniques for inducing both subacute and acute hindlimb ischemia, as described here. In addition to the variation inherent in animal studies, other factors can introduce variability in laser Doppler perfusion imaging (LDPI), including the type of anesthesia, position of the animal (supine vs. prone), and body temperature (see Figure 3). In addition, the subacut...
Discussion
Perhaps the most challenging step in this procedure is the separation of the femoral artery from the femoral vein. The larger diameter and thinner walls of the femoral vein compared to those of the artery increase its susceptibility to puncture and tearing during surgical manipulation. The likelihood of disrupting the vein can be reduced by keeping the wound moist using a sterile swab moistened with PBS. It is also important to ensure that all forceps are sharpened, aligned, and free of breaks in order to allow precise m...
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
This study was supported by NIH grants R21HL118661, R56HL124444, and R01HL124444 to CDK, and by NIH grants R00HL103797 and R01HL125695 to JMM.
Materials
| Name | Company | Catalog Number | Comments |
| Dumont #5/45 Forceps | Fine Science Tools | 11251-35 | Dumoxel |
| Dumont Style 5 Mini Forceps | Fine Science Tools | 11200-14 | Inox |
| Extra Fine Bonn Scissors | Fine Science Tools | 14084-08 | |
| 7 - 0 Silk Suture | Sharpoint | DA-2527N | |
| 5 - 0 Coated Vicryl Suture | Ethicon | J463G | |
| Graefe Forceps | Fine Science Tools | 11053-10 | |
| Vannas Spring Scissors | Fine Science Tools | 15000-03 | |
| Artifical Tears Ointment | Rugby Laboratories | 0536-6550-91 | |
| Surgical Tape | 3M | 1530-0 | |
| Fine Cotton Swabs | Contec | SC-4 | |
| Temperature Controller | Physitemp | TCAT-2DF | |
| Ameroid Constrictors | Research Instruments SW | MMC-0.25 x 1.00-SS | |
| Hot Bead Sterilizer | |||
| Deltaphase Isothermal Pad | Braintree Scientific | 39DP | |
| Needle Driver | Fine Science Tools | ||
| Phosphate Buffered Saline | Gibco | 10010-023 | |
| Moor LDPI | Moor Instruments | moorLDI2 | |
| moorLDI Measurement software | Moor Instruments | v. 6.0 | |
| Hair Removal Cream | Nair |
References
- Criqui, M. H., Aboyans, V. Epidemiology of peripheral artery disease. Circ. Res. 116, 1509-1526 (2015).
- Thukkani, A. K., Kinlay, S. Endovascular intervention for peripheral artery disease. Circ. Res. 116, 1599-1613 (2015).
- Vartanian, S. M., Conte, M. S. Surgical intervention for peripheral arterial disease. Circ. Res. 116, 1614-1628 (2015).
- Bonaca, M. P., Creager, M. A. Pharmacological treatment and current management of peripheral artery disease. Circ. Res. 116, 1579-1598 (2015).
- Conte, M. S., et al. Design and rationale of the PREVENT III clinical trial: edifoligide for the prevention of infrainguinal vein graft failure. Vasc Endovascular Surg. 39, 15-23 (2005).
- Pomposelli, F. B., et al. A decade of experience with dorsalis pedis artery bypass: analysis of outcome in more than 1000 cases. J. Vasc. Surg. 37, 307-315 (2003).
- Willigendael, E. M., et al. Smoking and the patency of lower extremity bypass grafts: a meta-analysis. J. Vasc. Surg. 42, 67-74 (2005).
- Schillinger, M., et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N. Engl. J. Med. 354, 1879-1888 (2006).
- Marmagkiolis, K., et al. 12-month primary patency rates of contemporary endovascular device therapy for femoro-popliteal occlusive disease in 6,024 patients: beyond balloon angioplasty. Catheter. Cardiovasc. Interv. 84, 555-564 (2014).
- Rosenfield, K., et al. Trial of a Paclitaxel-Coated Balloon for Femoropopliteal Artery Disease. N. Engl. J. Med. 373, 145-153 (2015).
- Tepe, G., et al. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 131, 495-502 (2015).
- Couffinhal, T., et al. Mouse model of angiogenesis. Am. J. Pathol. 152, 1667-1679 (1998).
- Niiyama, H., Huang, N. F., Rollins, M. D., Cooke, J. P. Murine model of hindlimb ischemia. J Vis Exp. , (2009).
- McClung, J. M., et al. Skeletal muscle-specific genetic determinants contribute to the differential strain-dependent effects of hindlimb ischemia in mice. Am. J. Pathol. 180, 2156-2169 (2012).
- Dokun, A. O., et al. A quantitative trait locus (LSq-1) on mouse chromosome 7 is linked to the absence of tissue loss after surgical hindlimb ischemia. Circulation. 117, 1207-1215 (2008).
- Rivard, A., et al. Age-dependent impairment of angiogenesis. Circulation. 99, 111-120 (1999).
- Hazarika, S., et al. Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus: differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ. Res. 101, 948-956 (2007).
- Couffinhal, T., et al. Impaired collateral vessel development associated with reduced expression of vascular endothelial growth factor in ApoE-/- mice. Circulation. 99, 3188-3198 (1999).
- Tang, G. L., Chang, D. S., Sarkar, R., Wang, R., Messina, L. M. The effect of gradual or acute arterial occlusion on skeletal muscle blood flow, arteriogenesis, and inflammation in rat hindlimb ischemia. J. Vasc. Surg. 41, 312-320 (2005).
- Yang, Y., et al. Cellular and molecular mechanism regulating blood flow recovery in acute versus gradual femoral artery occlusion are distinct in the mouse. J. Vasc. Surg. 48, 1546-1558 (2008).
- Litvak, J., Siderides, L. E., Vineberg, A. M. The experimental production of coronary artery insufficiency and occlusion. Am. Heart J. 53, 505-518 (1957).
- Bredee, J. J. An improved ameroid constrictor. Preliminary communication. J. Surg. Res. 9, 107-112 (1969).
- McClung, J. M., et al. Subacute limb ischemia induces skeletal muscle injury in genetically susceptible mice independent of vascular density. J. Vasc. Surg. , (2015).
- Hellingman, A. A., et al. Variations in surgical procedures for hind limb ischaemia mouse models result in differences in collateral formation. Eur. J. Vasc. Endovasc. Surg. 40, 796-803 (2010).
- Kochi, T., et al. Characterization of the arterial anatomy of the murine hindlimb: functional role in the design and understanding of ischemia models. PloS one. 8, e84047 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved