A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
The Preparation and Properties of Thermo-reversibly Cross-linked Rubber Via Diels-Alder Chemistry
In This Article
Summary
A simple two-step approach involving rubber modification and cross-linking yields fully reworkable, elastic rubber products.
Abstract
A method for using Diels Alder thermo-reversible chemistry as cross-linking tool for rubber products is demonstrated. In this work, a commercial ethylene-propylene rubber, grafted with maleic anhydride, is thermo-reversibly cross-linked in two steps. The pending anhydride moieties are first modified with furfurylamine to graft furan groups to the rubber backbone. These pendant furan groups are then cross-linked with a bis-maleimide via a Diels-Alder coupling reaction. Both reactions can be performed under a broad range of experimental conditions and can easily be applied on a large scale. The material properties of the resulting Diels-Alder cross-linked rubbers are similar to a peroxide-cured ethylene/propylene/diene rubber (EPDM) reference. The cross-links break at elevated temperatures (> 150 °C) via the retro-Diels-Alder reaction and can be reformed by thermal annealing at lower temperatures (50-70 °C). Reversibility of the system was proven with infrared spectroscopy, solubility tests and mechanical properties. Recyclability of the material was also shown in a practical way, i.e., by cutting a cross-linked sample into small parts and compression molding them into new samples displaying comparable mechanical properties, which is not possible for conventionally cross-linked rubbers.
Introduction
Sulfur vulcanization and peroxide curing are currently the main industrial cross-linking techniques in the rubber industry, yielding irreversible chemical cross-links that prevent melt reprocessing.1, 2 A 'cradle to cradle' approach to recycle cross-linked rubbers requires a material that behaves similar to permanently cross-linked rubbers at service conditions, while having the processability and complete recyclability of a thermoplastic at high temperatures. An approach to achieve such recyclability uses rubbery networks with reversible cross-links that respond to an external stimulus, such as temperature (most feasible from the viewpoint of future industrial applications).3-5 The formation of these cross-links at relatively low service temperatures is required for good mechanical behavior of the rubber, while their cleavage at high temperatures (similar to processing temperature of original non-cross-linked compound) allows for recycling of the material.
Some specific materials can be reversibly cross-linked by making use of so-called dynamic covalent networks via polycondensation reactions6 or by so-called reversible network topology freezing via transesterification reactions.7-9 The disadvantage of these approaches is the necessity of designing and synthesizing new polymers rather than modifying existing, commercial rubbers that already have the desired properties. Techniques to thermo-reversibly cross-link rubbers involve hydrogen bonding, ionic interactions and covalent bonding such as via thermo-activated disulfide rearrangements.10-13 Recently, thermo-reversible cross-linking via Diels-Alder (DA) chemistry was developed.14-21 DA chemistry can be applied to a broad range of polymers and represents a popular choice, especially since the DA reaction allows for relatively fast kinetics and mild reaction conditions.17, 22-24 Their low coupling and high decoupling temperatures make furan and maleimide excellent candidates for reversible polymer cross-linking.18-20, 25-28
The aim of the present work is to provide a method for the use of DA chemistry as a thermo-reversible cross-linking tool for an industrial rubber product (Figure 1).5 First, the reactivity of saturated hydrocarbon elastomers, such as ethylene/propylene rubbers (EPM), has to be increased. A commercially relevant example that facilitates this is the peroxide-initiated free-radical grafting of maleic anhydride (MA).29-34 Secondly, a furan group can be grafted onto such a maleated EPM rubber by inserting furfurylamine (FFA) into the pendant anhydride to form an imide.35, 36 Finally, the furan moieties that are thus attached to the rubber backbone can then participate in thermo-reversible DA chemistry as an electron-rich diene.25, 37 The electron-poor bis-maleimide (BM) is a suitable dienophile for this cross-linking reaction.19, 26, 38

Figure 1. Reaction scheme. Furan grafting and bismaleimide cross-linking of EPM-g-MA rubber (reprinted with permission from 5). Please click here to view a larger version of this figure.
Protocol
1. Rubber Modification
- Prepare the maleated EPM (EPM-g-MA, 49 wt% ethylene, 2.1 wt% MA, Mn = 50 kg/mol, PDI = 2.0) rubber and furfurylamine (FFA) before starting the experiment as indicated in steps 1.1.1-1.1.4.5
- Dry the EPM-g-MA rubber in a vacuum oven for one hour at 175 °C to convert present di-acid into anhydride.11
- Compression mold a 0.1 mm thick rubber film in a hot press for 10 min at 150 °C and 100 bar.
- Record a transmission infrared spectrum of the resulting film after placing it in a KBr tablet holder.
NOTE: The conversion of the hydrolyzed di-acid into anhydride is complete if the typical carboxylic acid peak ( = 1,710 cm-1) is absent and the characteristic cyclic anhydride peak (
= 1,710 cm-1) is absent and the characteristic cyclic anhydride peak (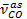 = 1,856 cm-1) is present.5
= 1,856 cm-1) is present.5 - Using standard distillation glassware, distill 2.8 g FFA (boiling point = 145 °C, 28.9 mmol; 3.0 eq. based on MA content in EPM-g-MA) under atmospheric pressure.
- Prepare a 10 wt% rubber solution by weighing 45.0 g of EPM-g-MA rubber (9.6 mmol MA) and dissolving it in 500 ml tetrahydrofuran (THF) at 23 °C in a closed beaker under vigorous stirring.
- Add the 2.8 g freshly distilled FFA to the 10 wt% rubber solution.
- Stir the reaction mixture in a closed system at 23 °C for at least 1 hr.
- Precipitate the reaction mixture by pouring it slowly into a tenfold (5 L) of acetone under mechanical stirring, yielding the polymer product as white threads that are easily fished out of the beaker using large tweezers.
- Dry the collected product (EPM-g-furan) to constant weight in a vacuum oven at 35 °C (this takes approximately 1 day).
- Compression mold the resulting, slightly yellowish product in a mold between two metal plates in a hot press for 10 min at 175 °C and 100 bar to convert the intermediate maleamic acid product into the imide product.
- Cut the resulting plaque of rubber in small pieces (0.05 g) with scissors and wash them thoroughly by immersing them in acetone to remove any unreacted FFA.
- Record a transmission infrared spectrum of the product in a KBr tablet holder after compression molding it into a 0.1 mm thick film.5
NOTE: The absence of any remaining amide-acid can be deduced from the absence of a peak at 1,530 cm-1.39, 40 The most illustrative indication for the successful modification are the nearly complete disappearance of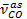 at 1,856 cm-1 of the anhydride and the appearance of
at 1,856 cm-1 of the anhydride and the appearance of  at 1,710 cm-1 and the C-N stretching vibration (
at 1,710 cm-1 and the C-N stretching vibration (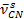 = 1,378 cm-1) of the maleimide.
= 1,378 cm-1) of the maleimide. - Determine the reaction conversion of EPM-g-MA to EPM-g-furan from the decrease in absorbance of the C=O symmetrical stretch vibration of the anhydride groups (
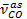 = 1,856 cm-1) by integrating the areas under the individual infrared (FT-IR) peaks after deconvolution (R2 > 0.95).5
= 1,856 cm-1) by integrating the areas under the individual infrared (FT-IR) peaks after deconvolution (R2 > 0.95).5
NOTE: The methyl rocking vibration (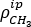 = 723 cm-1), originating from the EPM backbone, remains unchanged upon modification and can be used as an internal reference.
= 723 cm-1), originating from the EPM backbone, remains unchanged upon modification and can be used as an internal reference. - Determine the modification conversions by performing Elemental Analysis (EA) for N, C and H on the washed and dried rubber samples.5
NOTE: The molar contents can be derived from the measured mass percentages. The molar nitrogen content in EPM-g-furan is equal to that of the grafted furan groups. The conversion can be calculated by comparing the molar ratio of MA-grafted monomer to the non-grafted EPM monomers in the EPM-g-MA precursor (7.69 x 10-3) with the molar ratio N:EPM of the EPM-g-furan sample. - Measure the Shore A Hardness of the compression molded samples at least 10 times pressing a durometer onto a rubber sample, covering the entire cylindrical surface of the Durometer.5
NOTE: Samples with a thickness of 2 ± 0.1 mm should be used for these tests. - Measure the elongation (at break) and the ultimate tensile strength by performing tensile tests on samples of approximately 1 mm thick and 5 mm wide using a clamp length of 15 mm and a strain rate of 500 ± 50 mm/min. Determine the Young's modulus from the initial slope of the resulting stress-strain curves.
NOTE: For each measurement, test 10 samples and exclude two outliers with the highest and the lowest values. - Determine the compression set at 23 °C by compressing cylindrical samples with a thickness of 6 ± 0.1 mm (t0) and a diameter of 13 ± 0.1 mm between to metal plates to 3/4th of their initial thickness (tn) for 70 hr, let them relax at 50 °C for ½ hr and measure the thickness (ti).
NOTE: The compression set value can be determined from (t0-ti)/(t0-tn).
2. Diels-Alder Cross-linking and Reprocessing
- Before the experiment, synthesize the aliphatic bismaleimide (BM) from didodecylamine and maleic anhydride (MA) according to a reported procedure.41
- Weigh 40.0 g of EPM-g-furan rubber (8.6 mmol furan content) and 0.04 g phenolic anti-oxidant (octadecyl-1-[3,5-di-tert-butyl-4-hydroxyphenyl]propionate) and dissolve them in a large, closed beaker with 500 ml THF at 23 °C.
- Add 1.48 g of the aliphatic bismaleimide (4.3 mmol, 0.5 eq. based on furan content in EPM-g-furan) to beaker with the 10 wt% solution.
- Stir the reaction mixture for at least 1 hr at 50 °C in the closed beaker, then remove the cap to open the system to evaporate the solvent. Evaporation of the THF can also be performed using a rotary evaporator.
- Dry the collected product to constant weight in a vacuum oven at 35 °C.
- Compression mold the product for 30 min at 175 °C and 100 bar.
- Anneal the resulting product by storing it in an oven at 50 °C for at least three days.
- Cut the resulting plaque of rubber in small pieces (0.05 g) using scissors and wash them thoroughly by immersing them in acetone to remove any unreacted components and compression mold it into a 0.1 mm thick film.
- Record an transmission infrared spectrum of the resulting film in a KBr tablet holder, using the same set-up as described for 1.9.1.5
- Determine the cross-linking conversion from relative decrease in the C-O-C symmetric stretch vibration of the furan rings (
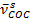 = 1,013 cm-1) as described in 1.10.5
= 1,013 cm-1) as described in 1.10.5 - Determine the cross-linking conversion by performing EA for N, C and H on the washed and dried rubber samples.5
- Determine the Shore A hardness, Young's modulus, the elongation at break, the ultimate tensile strength and compression set at 23 °C in the same way as described in 1.12-14.
- Reprocess the samples after testing by cutting them into small pieces using scissors (± 50 mm3) and compression molding these under the same conditions into new, homogeneous samples with the same dimensions.
3. Peroxide and Sulfur Curing of ENB-EPDM
- Heat an internal batch mixer to 70 °C and let it rotate at 50 rpm.
NOTE: An initial conditioning step by flushing the chamber with nitrogen gives better control of the cross-linking processes. - Feed 18.1 g of ENB-EPDM (48 wt% ethylene, 5.5 wt% ENB) to the internal batch mixer to reach a 70% fill factor and mix for 2 min to yield a homogeneous melt.
- Feed 1.25 phr of peroxide di(tert-butylperoxy-isopropyl) benzene) or 1.88 phr of a 80% pure, standard, semi-efficient sulfur vulcanization system and mix it with the rubber for 3 min at 70 °C.
- Compression mold the resulting compound in a hot press for 30 min at 175 °C and 100 bar to cure it.
- Determine the Shore A hardness, Young's modulus, the elongation at break and the ultimate tensile strength compression set in the same way as described in 1.12-14.
4. Cross-link Density Determination
- Cut a piece of compression molded, cross-linked rubber of approximately 50 mg using scissors.
- Determine the initial weight of the rubber sample precisely by weighing it in a 20 ml glass vial (W0).
- Immerse the weighed rubber in 15 ml of decalin.
- Let the rubber swell in the decalin until its weight does not increase anymore and equilibrium swelling is reached (approximately 3 days).
- Carefully take the swollen sample out of the vial and carefully dab the surface with a paper tissue to remove any solvent from the surface without squeezing it.
- Weigh the swollen rubber sample in a new sample vial (W1).
- Dry the swollen sample in a vacuum oven at 80 °C until a constant weight is reached and determine the sample's dry weight (W2).
- Obtain the gel content from W2/W0 x 100%
- Determine the cross-link density ([XLD] in mol/cm3) using the Flory-Rehner equation 42, 43, [XLD]= (ln(1-VR)+VR +χVR2)/(2VS(0.5VR-VR1/3) ) with VS the molar volume of the solvent (decalin: 154 ml/mol at 23 °C), χ the interaction parameter (decalin-EPDM: 0.121 + 0.278VR44) and VR the volume fraction of rubber in swollen sample that can be determined from W2/(W2+(W1-W2)∙ρEPM-g-furan/ρdecalin) with the densities (ρ) being 860 kg/m3 for EPM-g-furan and 896 kg/m3 for decalin, respectively.
Results
The successful modification of EPM-g-MA into EPM-g-furan and the cross-linking with the bismaleimide is shown by Fourier transform infrared spectrometry (FTIR) (Figure 2). The presence of furan groups in the EPM-g-furan product can be deduced from the splitting of the CC aliphatic stretching peak (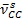 = 1,050 cm-1) into two furan peaks (
= 1,050 cm-1) into two furan peaks (
Discussion
A commercial EPM-g-MA rubber was thermo-reversibly cross-linked in a simple two-step approach. The maleated rubber was first modified with FFA to graft furan groups onto the rubber backbone. The resulting pending furans show reactivity as Diels-Alder dienes. An aliphatic BM was used as cross-linking agent, resulting in a thermo-reversible bridge between two furan moieties. Both reactions were successful with good conversions (> 80%) according to infrared spectroscopy, elemental analysis. Cross-linking was shown by sw...
Disclosures
The author Martin van Duin is an employee of LANXESS Elastomers B.V. that produces EPM-g-MA rubbers used in this Article.
Acknowledgements
This research forms part of the research program of the Dutch Polymer Institute, project #749.
Materials
| Name | Company | Catalog Number | Comments |
| ENB-EPDM | LANXESS Elastomers B.V. | Keltan 8550C | |
| EPM-g-MA | LANXESS Elastomers B.V. | Keltan DE5005 | Vacuum oven for one hour at 175 °C |
| furfurylamine | Sigma-Aldrich | F20009 | Freshly distillated before use |
| di-dodecylamine | Sigma-Aldrich | 36784 | |
| maleic anhydride | Sigma-Aldrich | M0357 | |
| octadecyl-1-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate | Sigma-Aldrich | 367079 | |
| bis(tert-butylperoxy-iso-propyl) benzene | Sigma-Aldrich | 531685 | |
| tetrahydrofuran | Sigma-Aldrich | 401757 | |
| decalin | Sigma-Aldrich | 294772 | |
| acetone | Sigma-Aldrich | 320110 |
References
- Myhre, M., MacKillop, D. A. Rubber recycling. Rubber Chem Technol. 75 (3), 429-474 (2002).
- Baranwal, K. C., Stephens, H. L. . Basic Elastomer Technology. , (2001).
- Such, G. K., Johnston, A. P. R., Liang, K., Caruso, F. Synthesis and functionalization of nanoengineered materials using click chemistry. Prog Polym Sci. 37 (7), 985-1003 (2012).
- Kloxin, C. J., Scott, T. F., Adzima, B. J., Bowman, C. N. Covalent Adaptable Networks (CANS): A Unique Paradigm in Cross-Linked Polymers. Macromol. 43 (6), 2643-2653 (2010).
- Polgar, L. M., van Duin, M., Broekhuis, A. A., Picchioni, F. The use of Diels-Alder chemistry for thermo-reversible cross-linking of rubbers: the next step towards recycling of rubber products. Macromol. 48 (19), 7096-7105 (2015).
- Garcia, J. M., et al. Recyclable, strong thermosets and organogels via paraformaldehyde condensation with diamines. Sci. 344 (6185), 732-735 (2014).
- Montarnal, D., Capelot, M., Tournilhac, F., Leibler, L. Silica-like malleable materials from permanent organic networks. Sci. 334 (6058), 965-968 (2011).
- Capelot, M., Montarnal, D., Tournilhac, F., Leibler, L. Metal-catalyzed transesterification for healing and assembling of thermosets. J Am Chem Soc. 134 (18), 7664-7667 (2012).
- Cordier, P., Tournilhac, F., Soulie-Ziakovic, C., Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature. 451 (7181), 977-980 (2008).
- Imbernon, L., Oikonomou, E. K., Norvez, S., Leibler, L. Chemically crosslinked yet reprocessable epoxidized natural rubber via thermo-activated disulfide rearrangements. Polym Chem. 6 (23), 4271-4278 (2015).
- Van der Mee, M. A. J., Goossens, J. G. P., van Duin, M. Thermoreversible cross-linking of maleated ethylene/propylene copolymers with diamines and amino-alcohols. Polym. 49 (5), 1239-1248 (2008).
- Van der Mee, M. A. J., Goossens, J. G. P., Van Duin, M. Thermoreversible covalent crosslinking of maleated ethylene/propylene copolymers with diols. J Polym Sci A-Polym Chem. 46 (5), 1810-1825 (2008).
- Das, A., et al. Ionic modification turns commercial rubber into a self-healing material. Acs Appl Mater Interf. 7 (37), 20623-20630 (2015).
- Gandini, A. The furan/maleimide Diels-Alder reaction: A versatile click-unclick tool in macromolecular synthesis. Prog Polym Sci. 38 (1), 1-29 (2013).
- Toncelli, C., De Reus, D. C., Picchioni, F., Broekhuis, A. A. Properties of reversible Diels-Alder furan/maleimide polymer networks as function of crosslink density. Macromol Chem Phys. 213 (2), 157-165 (2012).
- Tian, Q., Rong, M. Z., Zhang, M. Q., Yuan, Y. C. Synthesis and characterization of epoxy with improved thermal remendability based on Diels-Alder reaction. Polym Int. 59 (10), 1339-1345 (2010).
- Franc, G., Kakkar, A. K. Diels-Alder "click" chemistry in designing dendritic macromolecules. Chem-a Eur J. 15 (23), 5630-5639 (2009).
- Goiti, E., Huglin, M. B., Rego, J. M. Thermal breakdown by the retro Diels-Alder reaction of crosslinking in poly[styrene-co-(furfuryl methacrylate). Macromol Rapid Comm. 24 (11), 692-696 (2003).
- Gheneim, R., Perez-Berumen, C., Gandini, A. Diels-Alder reactions with novel polymeric dienes and dienophiles: Synthesis of reversibly cross-linked elastomers. Macromol. 35 (19), 7246-7253 (2002).
- Moustafa, M. M. A. R., Gillies, E. R. Rubber functionalization by Diels-Alder chemistry: from cross-linking to multifunctional graft copolymer synthesis. Macromol. 46 (15), 6024-6030 (2013).
- Scheltjens, G., Diaz, M. M., Brancart, J., Van Assche, G., Van Mele, B. A self-healing polymer network based on reversible covalent bonding. React Funct Polym. 73 (2), 413-420 (2013).
- Gandini, A., Silvestre, A. J. D., Coelho, D. Reversible click chemistry at the service of macromolecular materials. Polym Chem. 2 (8), 1713-1719 (2011).
- Nandivada, H., Jiang, X., Lahann, J. Click chemistry: Versatility and control in the hands of materials scientists. Adv Mater. 19 (17), 2197-2208 (2007).
- Chen, X. X., et al. A thermally re-mendable cross-linked polymeric material. Sci. 295 (5560), 1698-1702 (2002).
- Laita, H., Boufi, S., Gandini, A. The application of the Diels-Alder reaction to polymers bearing furan moieties .1. Reactions with maleimides. Eur Polym J. 33 (8), 1203-1211 (1997).
- Gandini, A., Coelho, D., Silvestre, A. J. D. Reversible click chemistry at the service of macromolecular materials. Part 1: Kinetics of the Diels-Alder reaction applied to furan-maleimide model compounds and linear polymerizations. Eur Polym J. 44 (12), 4029-4036 (2008).
- Ax, J., Wenz, G. Thermoreversible networks by Diels-Alder Reaction of cellulose furoates with bismaleimides. Macromol Chem Phys. 213 (2), 182-186 (2012).
- Canary, S. A., Stevens, M. P. Thermally reversible cross-linking of polystyrene via the furan-maleimide Diels-Alder reaction. J Polym Sci A-Polym Chem. 30 (8), 1755-1760 (1992).
- Burlett, D. J., Lindt, J. T. Reactive processing of rubbers. Rubber Chem Technol. 66 (3), 411-434 (1993).
- Saelao, J., Phinyocheep, P. Influence of styrene on grafting efficiency of maleic anhydride onto natural rubber. J Appl Polym Sci. 95 (1), 28-38 (2005).
- Guldogan, Y., Egri, S., Rzaev, Z. M. O., Piskin, E. Comparison of maleic anhydride grafting onto powder and granular polypropylene in the melt by reactive extrusion. J Appl Polym Sci. 92 (6), 3675-3684 (2004).
- Van Duin, M. Grafting of polyolefins with maleic anhydride: Alchemy or technology. Macromol Symp. 202, 1-10 (2003).
- Barra, G. M. O., Crespo, J. S., Bertolino, J. R., Soldi, V., Pires, A. T. N. Maleic anhydride grafting on EPDM: Qualitative and quantitative determination. J Braz Chem Soc. 10 (1), 31-34 (1999).
- Oostenbrink, A. J., Gaymans, R. J. Maleic-anhydride grafting on epdm rubber in the melt. Polym. 33 (14), 3086-3088 (1992).
- Schmidt, U., Zschoche, S., Werner, C. Modification of poly(octadecene-alt-maleic anhydride) films by reaction with functional amines. J Appl Polym Sci. 87 (8), 1255-1266 (2003).
- Vermeesch, I., Groeninckx, G. Chemical modification of poly(styrene-co-maleic anhydride) with primary N-alkylamines by reactive extrusion. J Appl Polym Sci. 53 (10), 1365-1373 (1994).
- Zhang, Y., Broekhuis, A. A., Picchioni, F. Thermally self-healing polymeric materials: the next step to recycling thermoset polymers. Macromol. 42 (6), 1906-1912 (2009).
- Gousse, C., Gandini, A., Hodge, P. Application of the Diels-Alder reaction to polymers bearing furan moieties. 2. Diels-Alder and retro-Diels-Alder reactions involving furan rings in some styrene copolymers. Macromol. 31 (2), (1998).
- Mikroyannidis, J. A. Synthesis and Diels-Alder polymerization of furfurylidene and furfuryl-substituted maleamic acids. J Polym Sci A-Polym Chem. 30 (1), 125-132 (1992).
- Kossmehl, G., Nagel, H., Pahl, A. Cross-linking reactions on polyamides by bis- and tris(maleimide)s. Angew Makromol Chem. 227 (1), 139-157 (1995).
- Liu, X., et al. Kinetic study of Diels-Alder reaction involving in maleimide-furan compounds and linear polyurethane. Polym Bull. 70 (8), 2319-2335 (2013).
- Stamboliyska, B. A., Binev, Y. I., Radomirska, V. B., Tsenov, J. A., Juchnovski, I. N. IR spectra and structure of 2,5-pyrrolidinedione (succinimide) and of its nitranion: experimental and ab initio MO studies. J Molec Struct. 516 (2-3), 237-245 (2000).
- Sombatsompop, N., Kumnuantip, C. Rheology, cure characteristics, physical and mechanical properties of tire tread reclaimed rubber/natural rubber compounds. J Appl Polym Sci. 87 (10), 1723-1731 (2003).
- Kim, J. K., Lee, S. H. New technology of crumb rubber compounding for recycling of waste tires. J Appl Polym Sci. 78 (8), 1573-1577 (2000).
- Dikland, H. G., van Duin, A. Miscibility of EPM-EPDM blends. Rubber Chem Technol. 76 (2), 495-506 (2003).
- Klots, T. D., Chirico, R. D., Steele, W. V. Complete vapor-phase assignment for the fundamental vibrations of furan, pyrrole and thiophene. Spectrochim Acta A-Mol Biomol Spectr. 50 (4), 765-795 (1994).
- Litvinov, V. M., Barendswaard, W., van Duin, M. The density of chemical crosslinks and chain entanglements in unfilled EPDM vulcanizates as studied with low resolution, solid state 1H-NMR. Rubber Chem Technol. 71 (1), 105-118 (1998).
- Orza, R. A., Magusin, P. C. M. M., Litvinov, V. M., van Duin, M., Michels, M. A. J. Solid-state 1H-NMR study on chemical cross-links, chain entanglements, and network heterogeneity in peroxide-cured EPDM rubbers. Macromol. 40 (25), 8999-9008 (2007).
- Henssler, J. T., Matzger, A. J. Regiochemical effects of furan substitution on the electronic properties and solid-state structure of partial fused-ring oligothiophenes. J Org Chem. 77 (20), 9298-9303 (2012).
- Hofmann, W. . Rubber Technology Handbook. , (1989).
- Chen, Y., Xu, C. Stress-strain behaviors and crosslinked networks Studies of natural rubber-zinc dimethacrylate composites. J Macromol Sci B-Phys. 51 (7), 1384-1400 (2012).
- Pritchard, R. H., Terentjev, E. M. Swelling and de-swelling of gels under external elastic deformation. Polym. 54 (26), 6954-6960 (2013).
- Tizard, G. A., Dillard, D. A., Norris, A. W., Shephard, N. Development of a high precision method to characterize Poisson's ratios of encapsulant gels using a flat disk configuration. Exp Mech. 52 (9), 1397-1405 (2012).
- Dijkhuis, K. A. J., Babu, I., Lopulissa, J. S., Noordermeer, J. W. M., Dierkes, W. K. A mechanistic approach to EPDM devulcanization. Rubber Chem. Technol. 81 (2), 865-880 (2008).
- Sutanto, P., Picchioni, E., Janssen, L. P. B. M., Dijkhuis, K. A. J., Dierkes, W. K., Noordermeer, J. W. M. State of the art: Recycling of EPDM rubber vulcanizates. Int Polym Proc. 21 (2), (2006).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved