A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Capillary Electrophoresis to Monitor Peptide Grafting onto Chitosan Films in Real Time
In This Article
Summary
Free solution capillary electrophoresis is a fast, cheap and robust analytical method that enables the quantitative monitoring of chemical reactions in real time. Its utility for rapid, convenient and precise analysis is demonstrated here through analysis of covalent peptide grafting onto chitosan films for improved cell adhesion.
Abstract
Free-solution capillary electrophoresis (CE) separates analytes, generally charged compounds in solution through the application of an electric field. Compared to other analytical separation techniques, such as chromatography, CE is cheap, robust and effectively requires no sample preparation (for a number of complex natural matrices or polymeric samples). CE is fast and can be used to follow the evolution of mixtures in real time (e.g., chemical reaction kinetics), as the signals observed for the separated compounds are directly proportional to their quantity in solution.
Here, the efficiency of CE is demonstrated for monitoring the covalent grafting of peptides onto chitosan films for subsequent biomedical applications. Chitosan's antimicrobial and biocompatible properties make it an attractive material for biomedical applications such as cell growth substrates. Covalently grafting the peptide RGDS (arginine - glycine - aspartic acid - serine) onto the surface of chitosan films aims at improving cell attachment. Historically, chromatography and amino acid analysis have been used to provide a direct measurement of the amount of grafted peptide. However, the fast separation and absence of sample preparation provided by CE enables equally accurate yet real-time monitoring of the peptide grafting process. CE is able to separate and quantify the different components of the reaction mixture: the (non-grafted) peptide and the chemical coupling agents. In this way the use of CE results in improved films for downstream applications.
The chitosan films were characterized through solid-state NMR (nuclear magnetic resonance) spectroscopy. This technique is more time-consuming and cannot be applied in real time, but yields a direct measurement of the peptide and thus validates the CE technique.
Introduction
Free solution capillary electrophoresis (CE) is a technique that separates compounds in solutions based on their charge-to-friction ratio1,2. Charge-to-size ratio is often mentioned in the literature, but this simplification does not apply to polyelectrolytes, including polypeptides in this work, and was also shown not to be appropriate for small organic molecules3. CE differs from other separation techniques in that it does not have a stationary phase, only a background electrolyte (usually a buffer). This allows the technique to be robust in its ability to analyze a large range of samples with complex matrices4 such as plant fibers5, fermentation brews6 grafting onto synthetic polymers7, food samples8, and hardly soluble peptides9 without tedious sample preparation and purification. This is especially significant for complex polyelectrolytes which have dissolution issues (such as chitosan10 and gellan gum11) and therefore exist as aggregated or precipitated in solution and have been successfully analyzed without sample filtration. Further, the analysis of sugars in breakfast cereals involved injecting samples with particles of breakfast cereal samples precipitated in water8. This also extends to the analysis of branched polyelectrolytes or copolymers12,13. Extensive work has also been completed in the development of CE techniques specifically for the analysis of proteins for proteomics14, chiral separation of natural or synthetic peptides15 and microchip separations of proteins and peptides16. Since the separation and analysis take place in a capillary, only small volumes of sample and solvents are used which enables CE to have a lower running cost than other separation techniques including chromatography5,6,17. Since the separation by CE is fast, it allows the monitoring of reaction kinetics. This was demonstrated in the case of the grafting of peptides onto chitosan films for improved cell adhesion18.
Chitosan is a polysaccharide derived from the N-deacetylation of chitin. Chitosan films can be used for various biomedical applications such as bioadhesives19 and cell growth substrates18,20, due to chitosan's biocompatibility21. Cell attachment to specific extracellular matrix proteins, such as fibronectin, collagens and laminin, is directly linked to the survival of the cells22. Notably, different cell types often require attachment to different extracellular matrix proteins for survival and proper function. Cell attachment to chitosan films was shown to be enhanced through the grafting of fibronectin23; however, preparation, purification and grafting of such large proteins is not economically viable. Alternately a range of small peptides have been shown to be able to mimic the properties of large extracellular matrix proteins. For example, peptides such as the fibronectin mimetics RGD (arginine - glycine - aspartic acid) and RGDS (arginine - glycine - aspartic acid - serine) have been used to facilitate and increase cell attachment24. Covalent grafting of RGDS onto chitosan films resulted in improved cell attachment for cells known to attach to fibronectin in vivo18. Substituting larger proteins likes fibronectin with smaller peptides that have the same functionality provides a significant cost reduction.
Here, peptide grafting to chitosan was performed as previously published18. As previously demonstrated, this approach provides simple and efficient grafting by using the coupling agents EDC-HCl (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide) and NHS (N-hydroxysuccinimide) to functionalize the carboxylic acid of the RGDS to be grafted onto the chitosan film. Two advantages of this grafting method are that it does not require any modification of the chitosan or of the peptide, and it is undertaken in aqueous medium to maximize compatibility with future cell culture applications18,20. As the coupling agents and the peptide can be charged, CE is a suitable method for the analysis of the reaction kinetics. Importantly, analysis of the reaction kinetics via CE enables real-time monitoring of the grafting reaction, and thus enables both optimizing and quantifying the degree of grafting.
While it is not routinely necessary, the results of the CE analysis can be validated off-line by a direct measurement of the peptide grafting onto the chitosan films using solid-state NMR (nuclear magnetic resonance) spectroscopy25,26 to demonstrate the covalent grafting of the peptide onto the film18. However, compared with solid-state NMR spectroscopy, the real-time analysis provided by CE enables the quantification of the peptide consumption in real time and thus the ability to assess the kinetics of the reaction.
The above mentioned method is simple and allows the real-time analysis of peptide grafting onto chitosan films with indirect quantification of the extent of the grafting. The demonstrated method can be extended to the real time quantitative assessment of different chemical reactions as long as the reactants or the products to be analyzed can be charged.
Protocol
1. Preparation of Chitosan Films
- Weigh out 2 g of glacial acetic acid, complete to 100 ml with ultrapure water.
- Weigh out 1.7 g of chitosan powder, add 100 ml of the 2% m/m acetic acid aqueous solution. Stir for 5 days with stirring bar and magnetic stirring plate at room temperature either covered with aluminum foil or in the dark.
- Centrifuge the chitosan dispersion at 1,076 x g at 23 °C for 1 hr. Collect the supernatant with a syringe and discard the precipitate.
- For each film, aliquot 10 ml of the chitosan suspension into a 9 cm plastic Petri dish at room temperature. Leave the films covered to dry for at least 7 days.
- Using scissors cut the dry films into 1 x 1 cm squares. Note: The experiment can be paused at this stage.
2. Preparation of Phosphate-buffered Saline (PBS)
- Weigh out 8 g sodium chloride, 0.2 g potassium chloride, 1.44 g disodium hydrogen phosphate and 0.24 g potassium dihydrogen phosphate.
- Dissolve these weighed out chemicals in 800 ml of ultrapure water and titrate the solution with concentrated hydrochloric acid to pH 7.4.
Note: The experiment can be paused at this stage.
3. Preparation of 75 mM Sodium Borate Buffer at pH 9.2
- Weigh out 3.0915 g of boric acid. Dissolve it in 75 ml of ultrapure water.
- Titrate the boric acid solution to a pH of 9.2 with a sodium hydroxide solution at a concentration of 10 M or higher.
Caution: Concentrated sodium hydroxide solutions are corrosive and should be handled with gloves. - Complete with ultrapure water to obtain 100 ml of solution. This yields a 500 mM sodium borate buffer at pH 9.2.
- Dilute the 500 mM sodium borate buffer with ultrapure water to 75 mM sodium borate buffer. Note: The experiment can be paused at this stage.
4. Preparation of Chitosan Films for the Grafting Reaction
- Rinse 10 square chitosan films (1 x 1 cm) in 5 ml of PBS for 2 hr in a Petri dish at room temperature.
- During this time, prepare and validate the capillary electrophoresis instrument (step 5).
5. Preparation and Validation of the Capillary Electrophoresis Instrument
- Prepare a 43.5 cm bare fused silica capillary with an internal diameter of 50 µm (43.5 cm is the total length, the effective length to the detection window is typically 35 cm) by weakening the polymer outer coating of the capillary at the set length with a blunt utensil then snapping the capillary.
- Create a window for the capillary by using a lighter to burn the polymer coating at 8.5 cm from the inlet and after it cools wipe it clean with ethanol. Burn the coating of the capillary at each end for a few millimeters with a lighter, and after it cools wipe it clean with ethanol.
- Place capillary inside detection window and install it in the capillary cassette by placing it at equal lengths in the inlet and outlet and winding it around the spindles of the cassette. Then install the cassette in the capillary electrophoresis instrument.
- Set the parameters of the method for each separation. In the software menu select "method" then "edit the entire method". Set the temperature, time, voltage, and vials used for the separation (for example 25 °C, 10 min, 30 kV).
- In the pre-conditioning section, set the consecutive flushes: 10 min with 1 M sodium hydroxide (in water), 5 min with 0.1 M sodium hydroxide (in water), 5 min with ultrapure water and 5 min with 75 mM sodium borate buffer at pH 9.2 for the first method of a series of analyses.
- For the subsequent methods, set the set the consecutive flushes in the pre-conditioning section: 1 min with 1 M sodium hydroxide (in water), 5 min with 75 mM sodium borate buffer at pH 9.2.
- In the injection section, set parameters for a hydrodynamic injection with 30 mbar pressure for 10 sec for all methods. In the separation section, set the separation conditions to 30 kV at 25 °C for 9 min for all methods.
NOTE: Consult user manual of specific CE instrument as procedure for operating the CE instrument may vary between manufacturers. Prepare the 1 M sodium hydroxide solution on the day.
- Inject and separate a neutral internal standard (10 µl of 10% v/v dimethylsulfoxide (DMSO), in water diluted into 450 µl of 75 mM sodium borate buffer). Then inject and separate in the same way an oligoacrylate standard (dissolved in ultrapure water at 10 g∙L-1; see List of Materials) to check the validity of the capillary. Pause the sequence here until the grafting reaction is ready to start.
6. Grafting of RGDS onto Chitosan Film
- Weigh out the peptide (1 mg RGDS) and the coupling agents (3 mg EDC-HCl and 2 mg NHS).
- 2 hr after the start of the chitosan film soaking in PBS, dissolve the peptide and the coupling agents in 5 ml of PBS.
- Take a 50 µl aliquot of this solution. Add 2 µl of 10% v/v DMSO in water as an internal neutral standard to the aliquot. Analyze the aliquot with CE (see step 7).
- Remove the 5 ml of PBS used to rinse the chitosan films from the Petri dish. Add the 5 ml solution of peptide and coupling agents to the Petri dish containing the chitosan films.
- Cover the Petri dish with paraffin film and place it on an orbital shaker at room temperature. Take 50 µl aliquots of reaction media at set times.
NOTE: The total analysis time with CE is 15 min, thus an aliquot can be taken every 15 min (or every 30 min if two reactions are monitored in parallel, etc.).- Add 2 µl of 10% v/v DMSO in water as an internal neutral standard to each aliquot.
NOTE: Aliquots should be analyzed with CE as soon as they are taken (see step 7).
- Add 2 µl of 10% v/v DMSO in water as an internal neutral standard to each aliquot.
- After 4 hr of shaking and aliquot removal, remove the Petri dish from the shaker. Remove the reaction medium from the Petri dish. Add 5 ml of PBS to rinse the chitosan films.
- Remove the PBS from the Petri dish, rinse the chitosan film with ultrapure water and allow them to dry overnight. Remove the ultrapure water and store the films at -20 °C in a plastic Petri dish.
7. Monitoring of Grafting Reaction Using CE
- Inject and separate aliquots of reaction media immediately after removal from the Petri dish using the analysis conditions as in section 5.2.
- Upon completion of the separations rinse the capillary with ultrapure water for 10 min. Dry it through a flush with an empty vial (air) for 10 min.
NOTE: The experiment can be paused at this stage.
8. Data Treatment for CE
- Check the validity of each separation, by checking that both the current during the separation and the migration time of the electroosmotic mobility marker (DMSO in this case) are similar to the ones observed for the oligoacrylate standard separation.
NOTE: Up to 10-15% variation is acceptable from the expected current value of about 50 µA and migration time value of 1.3 min (electrophoretic mobility values should be used instead of migration times if a higher repeatability is required). - For each successful separation, export the raw data from the capillary electrophoresis software by selecting a specific data set, right clicking on export and selecting an appropriate signal.
- Convert the raw data recorded by the CE (presented as UV absorbance as a function of migration time). Convert the X-axis (migration time tm) into an electrophoretic mobility µ following Equation 1:
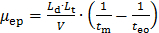 (1)
(1)
where Ld is the length to the detector, Lt is the total length of the capillary, V is the voltage, and teo is the migration time of a neutral specie (the DMSO internal standard in this case)27.
Convert the Y-axis of the raw data (absorbance in a.u.) to a distribution of electrophoretic mobilities W(µ) following Equation 2:28
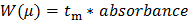 (2)
(2)
9. Additional Characterization of Peptide-grafted Films18
- Insert peptide-grafted chitosan films, rolled around themselves, in a 4 mm solid-state NMR rotor. Fill the rotor with phosphate-buffered saline to swell the films, and close the rotor. Wait for a few hours.
- Analyze the film with 13C NMR spectroscopy18.
Results
CE is well suited to monitoring the grafting of peptides (e.g., RGDS) onto chitosan films. Suitable coupling agents include EDC-HCl and NHS which activate the peptide to be grafted onto the chitosan (Figure 1). CE is able to separate the different molecules of interest from the reaction medium. To assign the peaks on the electropherogram, pure RGDS, EDC-HCl and NHS were dissolved, injected and separated separately. After the peak assignment, the reaction medium w...
Discussion
The simplicity of the protocol described here makes it ideally suited to widespread application. However, particular attention needs to be paid to of the following key steps.
Proper CE instrument preparation
It is important to separate a known standard immediately prior to the separation of unknown samples (as well as at the end of a series of separations) to check the validity of the capillary and instrument on the day. This standard can be an olig...
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgements
MG, MO'C and PC thank the Molecular Medicine Research Group at WSU for Research Seed Funding, as well as Michele Mason (WSU), Richard Wuhrer (Advanced Materials Characterisation Facility, AMCF, WSU) and Hervé Cottet (Montpellier) for discussions.
Materials
| Name | Company | Catalog Number | Comments |
| Water | Millipore | All water used in the experiment has to be of Milli-Q quality | |
| Chitosan powder (medium molecular weight) | Sigma-Aldrich | 448877 | lot MKBH1108V was used. Significant batch-to-batch variations occur with natural products such as polysaccharides |
| Acetic acid - Unilab | Ajax Finechem | 2-2.5L GL | laboratory reagent |
| Dimethylsulfoxide | Sigma-Aldrich | D4540 | laboratory reagent, slightly hazardous to skin, hazardous if ingested |
| Sodium hydroxide | Sigma-Aldrich | 221465 | laboratory reagent, corrosive |
| 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide | Sigma-Aldrich | D80002 | Irritant to skin |
| N-hydroxysuccinimide | Sigma-Aldrich | 130672 | Irritant to skin |
| Sodium chloride | Ajax Finechem | 466-500G | laboratory reagent |
| Potassium chloride - Univar | Ajax Finechem | 384-500G | analytical reagent, slight skin irritant |
| Disodium hydrogen phosphate - Unilab | Ajax Finechem | 1234-500G | laboratory reagent, slight skin irritant |
| Potassium dihydrogen phosphate - Univar | Ajax Finechem | 4745-500G | analytical reagent, slight skin irritant |
| Oligoacrylate standard | custom made | See reference for synthetic protocol: Castignolles, P.; Gaborieau, M.; Hilder, E. F.; Sprong, E.; Ferguson, C. J.; Gilbert, R. G. Macromol. Rapid Commun. 2006, 27, 42-46 | |
| Boric acid | BDH AnalR, Merck Pty Ltd | 10058 | Corrosive |
| Hydrochloric acid - Unilab | Ajax Finechem | A1367-2.5L | laboratory reagent, corrosivie |
| Fused silica tubing | Polymicro (Molex) | TSP050375 | Flexible fused silica capillary tubing with standard polyimide coating, 50 µm internal diameter, 363 µm outer diameter |
| Agilent 7100 CE | Agilent Technologies | G7100CE | Capillary electrophoresis instrument |
| Orbital shaker | IKA | KS260 | |
| Electronic balance | Mettler Toledo | MS204S | |
| Milli-Q Synthesis | Millipore | ZMQS5VF01 | Ultrapure water filtration system |
| Parafilm | Labtek | PM966 | Parrafin wax |
References
- Muthukumar, M. Theory of electrophoretic mobility of a polyelectrolyte in semidilute solutions of neutral polymers. Electrophoresis. 17, 1167-1172 (1996).
- Barrat, J. L., Joanny, J. F. . in Advances in Chemical Physics, Vol Xciv Vol. 94 Advances in Chemical Physics. , 1-66 (1996).
- Fu, S. L., Lucy, C. A. Prediction of electrophoretic mobilities. 1. Monoamines. Anal. Chem. 70, 173-181 (1998).
- Harvey, D. . Modern Analytical Chemistry. , (2000).
- Oliver, J. D., Gaborieau, M., Hilder, E. F., Castignolles, P. Simple and robust determination of monosaccharides in plant fibers in complex mixtures by capillary electrophoresis and high performance liquid chromatography. J. Chromatogr. A. 1291, 179-186 (2013).
- Oliver, J. D., Sutton, A. T., Karu, N., Phillips, M., Markham, J., Peiris, P., Hilder, E. F., Castignolles, P. Simple and robust monitoring of ethanol fermentations by capillary electrophoresis. Biotechnology and Applied Biochemistry. 62, 329-342 (2015).
- Thevarajah, J. J., Sutton, A. T., Maniego, A. R., Whitty, E. G., Harrisson, S., Cottet, H., Castignolles, P., Gaborieau, M. Quantifying the Heterogeneity of Chemical Structures in Complex Charged Polymers through the Dispersity of Their Distributions of Electrophoretic Mobilities or of Compositions. Anal. Chem. 88, 1674-1681 (2016).
- Toutounji, M. R., Van Leeuwen, M. P., Oliver, J. D., Shrestha, A. K., Castignolles, P., Gaborieau, M. Quantification of sugars in breakfast cereals using capillary electrophoresis. Carbohydr. Res. 408, 134-141 (2015).
- Miramon, H., Cavelier, F., Martinez, J., Cottet, H. Highly Resolutive Separations of Hardly Soluble Synthetic Polypeptides by Capillary Electrophoresis. Anal. Chem. 82, 394-399 (2010).
- Mnatsakanyan, M., Thevarajah, J. J., Roi, R. S., Lauto, A., Gaborieau, M., Castignolles, P. Separation of chitosan by degree of acetylation using simple free solution capillary electrophoresis. Anal. Bioanal. Chem. 405, 6873-6877 (2013).
- Taylor, D. L., Ferris, C. J., Maniego, A. R., Castignolles, P., in het Panhuis, M., Gaborieau, M. Characterization of Gellan Gum by Capillary Electrophoresis. Australian Journal of Chemistry. 65, 1156-1164 (2012).
- Thevarajah, J. J., Gaborieau, M., Castignolles, P. Separation and characterization of synthetic polyelectrolytes and polysaccharides with capillary electrophoresis. Adv. Chem. 2014, 798503 (2014).
- Sutton, A. T., Read, E., Maniego, A. R., Thevarajah, J., Marty, J. -. D., Destarac, M., Gaborieau, M., Castignolles, P. Purity of double hydrophilic block copolymers revealed by capillary electrophoresis in the critical conditions. J. Chromatogr. A. 1372, 187-195 (2014).
- Righetti, P. G., Sebastiano, R., Citterio, A. Capillary electrophoresis and isoelectric focusing in peptide and protein analysis. Proteomics. 13, 325-340 (2013).
- Ali, I., Al-Othman, Z. A., Al-Warthan, A., Asnin, L., Chudinov, A. Advances in chiral separations of small peptides by capillary electrophoresis and chromatography. J. Sep. Sci. 37, 2447-2466 (2014).
- Kasicka, V. Recent developments in capillary and microchip electroseparations of peptides (2011-2013). Electrophoresis. 35, 69-95 (2014).
- Taylor, D. L., Thevarajah, J. J., Narayan, D. K., Murphy, P., Mangala, M. M., Lim, S., Wuhrer, R., Lefay, C., O'Connor, M. D., Gaborieau, M., Castignolles, P. Real-time monitoring of peptide grafting onto chitosan films using capillary electrophoresis. Anal. Bioanal. Chem. 407, 2543-2555 (2015).
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 31, 603-632 (2006).
- Li, Z., Leung, M., Hopper, R., Ellenbogen, R., Zhang, M. Feeder-free self-renewal of human embryonic stem cells in 3D porous natural polymer scaffolds. Biomaterials. 31, 404-412 (2010).
- Domard, A. A perspective on 30 years research on chitin and chitosan. Carbohydr. Polym. 84, 696-703 (2011).
- Shekaran, A., Garcia, A. J. Nanoscale engineering of extracellular matrix-mimetic bioadhesive surfaces and implants for tissue engineering. Biochim. Biophys. Acta Gen. Subj. 1810, 350-360 (2011).
- Custodio, C. A., Alves, C. M., Reis, R. L., Mano, J. F. Immobilization of fibronectin in chitosan substrates improves cell adhesion and proliferation. J. Tissue Eng. Regen. Med. 4, 316-323 (2010).
- Boateng, S. Y., Lateef, S. S., Mosley, W., Hartman, T. J., Hanley, L., Russell, B. RGD and YIGSR synthetic peptides facilitate cellular adhesion identical to that of laminin and fibronectin but alter the physiology of neonatal cardiac myocytes. Am. J. Physiol. Cell Physiol. 288, C30-C38 (2005).
- Lefay, C., Guillaneuf, Y., Moreira, G., Thevarajah, J. J., Castignolles, P., Ziarelli, F., Bloch, E., Major, M., Charles, L., Gaborieau, M., Bertin, D., Gigmes, D. Heterogeneous modification of chitosan via nitroxide-mediated polymerization. Polym. Chem. 4, 322-328 (2013).
- Gartner, C., Lopez, B. L., Sierra, L., Graf, R., Spiess, H. W., Gaborieau, M. Interplay between Structure and Dynamics in Chitosan Films Investigated with Solid-State NMR, Dynamic Mechanical Analysis, and X-ray Diffraction. Biomacromolecules. 12, 1380-1386 (2011).
- Castignolles, P., Gaborieau, M., Hilder, E. F., Sprong, E., Ferguson, C. J., Gilbert, R. G. High resolution separation of oligo(acrylic acid) by capillary zone electrophoresis. Macromol. Rapid Commun. 27, 42-46 (2006).
- Chamieh, J., Martin, M., Cottet, H. Quantitative Analysis in Capillary Electrophoresis: Transformation of Raw Electropherograms into Continuous Distributions. Anal. Chem. 87, 1050-1057 (2015).
- Maniego, A. R., Ang, D., Guillaneuf, Y., Lefay, C., Gigmes, D., Aldrich-Wright, J. R., Gaborieau, M., Castignolles, P. Separation of poly(acrylic acid) salts according to topology using capillary electrophoresis in the critical conditions. Anal. Bioanal. Chem. 405, 9009-9020 (2013).
- Chung, T. W., Lu, Y. F., Wang, S. S., Lin, Y. S., Chu, S. H. Growth of human endothelial cells on photochemically grafted Gly-Arg-Gly-Asp (GRGD) chitosans. Biomaterials. 23, 4803-4809 (2002).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved