A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Phosphorus-31 Magnetic Resonance Spectroscopy: A Tool for Measuring In Vivo Mitochondrial Oxidative Phosphorylation Capacity in Human Skeletal Muscle
In This Article
Summary
This work demonstrates the feasibility of an in vivo phosphorus-31 magnetic resonance spectroscopy (31PMRS) technique to quantify mitochondrial oxidative phosphorylation (OXPHOS) capacity in human skeletal muscle.
Abstract
Skeletal muscle mitochondrial oxidative phosphorylation (OXPHOS) capacity, which is critically important in health and disease, can be measured in vivo and noninvasively in humans via phosphorus-31 magnetic resonance spectroscopy (31PMRS). However, the approach has not been widely adopted in translational and clinical research, with variations in methodology and limited guidance from the literature. Increased optimization, standardization, and dissemination of methods for in vivo 31PMRS would facilitate the development of targeted therapies to improve OXPHOS capacity and could ultimately favorably impact cardiovascular health. 31PMRS produces a noninvasive, in vivo measure of OXPHOS capacity in human skeletal muscle, as opposed to alternative measures obtained from explanted and potentially altered mitochondria via muscle biopsy. It relies upon only modest additional instrumentation beyond what is already in place on magnetic resonance scanners available for clinical and translational research at most institutions. In this work, we outline a method to measure in vivo skeletal muscle OXPHOS. The technique is demonstrated using a 1.5 Tesla whole-body MR scanner equipped with the suitable hardware and software for 31PMRS, and we explain a simple and robust protocol for in-magnet resistive exercise to rapidly fatigue the quadriceps muscle. Reproducibility and feasibility are demonstrated in volunteers as well as subjects over a wide range of functional capacities.
Introduction
The goal of this work is to outline a reproducible method to noninvasively measure in vivo skeletal muscle mitochondrial function in individuals possessing a wide range of abilities. Aberrant mitochondrial impairment is a hallmark of a wide range of metabolic syndromes and genetic diseases, from common conditions such as aging and diabetes to rare disorders such as Friedreich's ataxia.
Metabolic Syndrome and Mitochondrial Dysfunction
Metabolic syndrome has been shown to disrupt mitochondrial function, depress skeletal muscle OXPHOS, and lead to ectopic lipid storage in skeletal muscle1,2. As critical organelles regulating metabolic and energy homeostasis, mitochondria are implicated in the pathophysiology of obesity3,4, insulin resistance5, Type 2 Diabetes Mellitus (T2DM)6,7, diabetes-related micro-8,9,10,11 and macrovascular complications12,13, and non-alcoholic fatty liver disease (NAFLD)14,15,16, among others.Insulin resistance is characterized by profound changes in skeletal muscle mitochondrial activity, including decreased mitochondrial tricarboxylic acid (TCA) flux rate, ATP synthesis rate, and citrate synthase and NADH:O2 oxidoreductase activity5. One hypothesis is that these alterations could be due to the accumulation of free fatty acid (FFA) metabolites in the muscle, which are markedly augmented during obesity and other obesity-related diseases2,17. The exposure of muscle to elevated FFAs and lipid intermediates can decrease the expression of genes in the lipid oxidative pathway as well as the TCA cycle and electron-transport chain (ETC)18. This reduction in mitochondrial skeletal muscle OXPHOS capacity in the setting of a lipid overload is accompanied by a decline in the quantitative (content and biogenesis of mitochondria)19 and qualitative function of skeletal muscle mitochondria20. Exposing skeletal muscle and myocytes to FFAs leads to severe insulin resistance, and increased FFA uptake in muscle is associated with insulin resistance in both humans and rodents21. The lipid intermediates ceramide and diacylglycerol (DAG) have been shown to directly inhibit the insulin signaling pathway by altering the activity of kinases, such as protein kinase C and protein kinase B21. Therefore, lipid-derived molecules appear to play a prominent role in the development of skeletal muscle insulin resistance and T2DM. However, it remains unclear whether changes in mitochondrial capacity are a cause or a consequence of insulin resistance22.
Friedrich's Ataxia and Mitochondrial Dysfunction
Decreased OXPHOS can also arise from genetic defects. Friedrich's ataxia (FA), the most common form of hereditary ataxia, is a genetic disorder caused by a mutation in the frataxin (FXN) gene, resulting in intra-mitochondrial iron accumulation, reactive oxygen species production, and abnormalities of oxidative phosphorylation23,24,25,26. This important discovery has led to the development of targeted therapies, which aim to improve mitochondrial function at the sub-cellular level. Despite this understanding, there has been limited development of in vivo, reproducible biomarkers for FA clinical research. In fact, a critical barrier in the effective evaluation of targeted therapies in FA is the inability to track changes in mitochondrial function. Current functional measures, for example, can identify decreased cardiac output; however, they are incapable of determining the level at which the dysfunction occurs (Figure 1). The development of a reliable marker of mitochondrial function that can be used to identify and evaluate disease progression in Friedrich's ataxia is crucial to gauge the relevant mechanistic impact of targeted therapies.
Impaired OXPHOS and Cardiac Dysfunction
Aberrant mitochondrial function, either acquired or genetic, could contribute to the development or progression of cardiac dysfunction. Under the conditions of pressure overload and heart failure, the primary energy substrate preference switches from FFA to glucose. This is associated with decreased ETC activity and oxidative phosphorylation27. The pathophysiology of mitochondrial bioenergetics in cardiac dysfunction can be different depending on the primary origin of the mitochondrial defect. Diabetes and metabolic syndrome results in mitochondrial abnormalities in myocardium, such as impaired biogenesis and fatty acid metabolism, which lead to reduced substrate flexibility, energy efficiency, and eventually, diastolic dysfunction28,29. In FA, on the other hand, a frataxin deficiency results in significant mitochondrial iron accumulation in cardiomyocytes30,31. Iron accumulation leads to the production of free radicals via the Fenton reaction32 and increases the chance of free radical-induced cardiomyocyte damage. Intra-mitochondrial iron accumulation is also associated with an increased sensitivity to oxidative stress and a reduced oxidative capacity30,31. Iron accumulation and subsequent aberrant mitochondrial function, due to frataxin deficiency, may therefore be responsible for the impaired cardiac energetics and cardiomyopathy observed in FA33,34. It is also interesting to note that the reduced oxidative capacity in skeletal muscle mitochondria parallels the exercise intolerance and reduced metabolic capacity in heart failure (HF)35. Measurement of skeletal muscle OXPHOS capacity, as detailed herein, is readily implementable and robust; coupled with the significance of skeletal muscle OXPHOS in HF, these features make it an appealing biomarker in comprehensive studies of heart disease36.
Impaired OXPHOS and the accompanying cardiac dysfunction is not an inconsequential aspect of metabolic and mitochondrial disease. Subjects with diabetes and metabolic disease are at a higher risk of developing cardiovascular disease and have excess mortality after myocardial infarction (MI)37,38,39,40,41; over half of FA subjects have cardiomyopathy, and many die of cardiac arrhythmia or heart failure42. Therefore, quantification of reduced OXPHOS could not only allow for early detection and treatment of cardiac dysfunction, but it could also alleviate a major clinical burden in these diseases.
Targeted therapies to directly increase OXPHOS capacity is a promising area to improve the treatment of subjects, whether the cause of metabolic dysfunction is genetic or acquired. Currently, the development of novel targeted drugs that either alleviate abnormal mitochondrial function43 or correct the primary genetic defect44 can improve the deranged bioenergetics characteristic of FA. In the case of acquired mitochondrial dysfunction, increased physical activity can improve mitochondrial function 45,46,47.
31Phosphorous Magnetic Resonance Spectroscopy as a Non-invasive Biomarker of Mitochondrial Function
Regardless of the tested therapy, an integrated in vivo assessment of skeletal muscle bioenergetics is a crucial tool to assess the impact of targeted interventions, especially in subjects with severe exercise intolerance or the inability to undergo conventional metabolic testing. Magnetic resonance spectroscopy tuned to phosphorous (31PMRS), an endogenous nucleus found in various high-energy substrates within cells throughout the body, has been used to quantify mitochondrial oxidative capacity using a variety of approaches, including in-magnet exercise-recovery protocols and muscle stimulation protocols48. The exercise-recovery protocols rely upon a variety of apparatus ranging in complexity from MRI-compatible ergometers that regulate and measure workload to simple configurations of straps and pads allowing for burst-type resistive and quasi-static exercise. One of the primary goals of any of these protocols is to produce an energy imbalance for which the demand for adenosine triphosphate (ATP) is initially met through the enzymatic breakdown of phosphocreatine (PCr) through the creatine kinase reaction49. Upon cessation of exercise, the rate of ATP production is dominated by oxidative phosphorylation and represents the maximum in vivo capacity of the mitochondria50. Furthermore, OXPHOS during post-exercise recovery can be described by a first-order rate reaction51. The post-exercise recovery of PCr can therefore be quantified by the fitting of an exponential time constant (τPCr), with smaller values of τPCr representing greater capacities for oxidative ATP synthesis. Significant efforts have been made to validate 31PMRS against ex vivo and more direct measures of OXPHOS and demonstrate the potential clinical applicability of this technique52,53,54,55.
Notably, the protocol described in this work can be implemented on clinically-available scanners, and it has been widely validated as a noninvasive biomarker of mitochondrial function56. However, an exercise 31PMRS protocol optimized for application to individuals with varying severities of neuromuscular impairment or mobility has not been well established57. A well-defined, broadly-applicable exercise protocol and 31PMRS technique would be particularly useful in the evaluation of diseases with fundamental abnormalities in mitochondrial function.
Several prior studies have explored the applications of non-invasive techniques to quantify mitochondrial function in subjects. For instance, these techniques have shown impaired OXPHOS in subjects with type 2 diabetes36. Lodi et al. first tested the feasibility of PMRS techniques in subjects with FA and found that 1) the fundamental genetic defect in FA impairs skeletal muscle OXPHOS and 2) the number of GAA triplet repeats is inversely proportional to skeletal muscle OXPHOS33. More recently, Nachbauer et al. used PMRS as a secondary outcome measure in an FA drug trial with 7 subjects. PCr recovery times were significantly longer in subjects compared to controls, reaffirming Lodi's earlier work and indicating that the effects of aberrant frataxin expression in FA can result in a decline in mitochondrial capacity that is detectable using PMRS techniques58.
Reliable methods to adequately define in vivo skeletal muscle function in a feasible, cost-effective, and reproducible manner are critical to improving subject outcomes in a range of diseases that affect mitochondrial function.
This work outlines a robust procedure for obtaining in vivo maximum oxidative capacity of skeletal muscle using 31PMRS. The in-magnet exercise protocol is well tolerated by individuals spanning a wide range of physical and functional abilities and affords a simplified subject setup using inexpensive and widely-available equipment.
Protocol
This protocol is approved by and follows the guidelines of the Ohio State University Institutional Review Board for human subjects research. It is critically important that all procedures involving MR equipment are performed by adequately trained personnel adhering to the highest standards of MR safety59.
1. Materials and Preparation
- Ensure that all necessary materials are available prior to the experiment (Figure 2).
- Plug the 31P coil into the in-table coil connector at the end of the exam table closest to the bore. Place a large triangle foam cushion near the head of the MR exam table, but not directly on the 31P coil. Place a head pillow at the other end of the MR exam table, farthest from the bore, for subject comfort.
2. Subject Positioning (Figure 3a)
- Instruct the subject to lie supine, feet first on the MR table. Place a foam cushion under the knees to support the leg in a partially flexed position.
- Position the subject close to the right side of the table (the subject's right) in order to center the left thigh as closely to the magnet isocenter as possible, thus ensuring optimal B0 homogeneity in the thigh muscle under examination. Provide the subject with ear plugs and/or headphones.
- Position the 31P RF coil on the left quadriceps at approximately the midpoint between the patella and the femoral head, and secure to the leg using straps. Place the coil over the lateral portion of the leg, above the vastus lateralis.
- Secure the baby oil to the medial aspect of the thigh with the same straps used to secure the coil to the leg. This facilitates scan localization.
- Bind the subject's legs together with a strap placed below the coil and above the knee. Secure the subject's legs to the MR table with additional straps, one above the knee and one midway between the knee and ankle.
- Use the laser light guide to delineate the center of the coil and move the table to the magnet isocenter using this centering landmark.
3. Exercise Protocol
- Explain to the subject that the exercise protocol consists of three phases: an initial, baseline phase; a short, intense exercise phase; and a recovery phase.
- Instruct the subject to lie still and relax their leg muscles during the baseline and recovery phases of the spectroscopy acquisition in order to minimize motion artifacts.
- Provide a countdown to the subject indicating the start of exercise. At this point, have the subject initiate knee extension/flexion as forcefully and as rapidly as possible against the resistance of the straps.
NOTE: The quadriceps muscles are used to move the left lower leg up and down, until instructed to stop. - Terminate exercise after a 30% drop in the PCr peak height.
- Observe the PCr peak height in the acquisition viewer window, and also view it upon completion of the exercise sequence.
NOTE: A general guideline is that an approximate 30% drop in PCr peak height corresponds to a Pi peak that is 50% of the height of the PCr peak. If PCr depletion is not occurring rapidly enough to achieve a 30% drop during the exercise phase of the exam, encourage the subject to kick harder or faster while exercising.
NOTE: Cessation of exercise is determined by monitoring the PCr peak height and duration of exercise. This may result in slightly different durations of exercise in different patients and can be accounted for in the analysis.
- Observe the PCr peak height in the acquisition viewer window, and also view it upon completion of the exercise sequence.
4. Scan Protocol
- Acquire a tri-plane localizer to verify proper subject positioning and identify the location of the 31P coil.
NOTE: The localizer sequence begins automatically and centers at the indicated position using the laser light guide (step 2.9) - Acquire a second tri-plane localizer.
- Open the slice view on the first tri-plane localizer images.
NOTE: This process may be different for different software and hardware systems. - Center and rotate the slice orientation by left-clicking and holding on the slice group. Rotate the slice group. Ensure that the final orientation of slices matches with the position of the baby oil.
- In the sequence routine window, increase the number of slices to cover the entire leg in the axial and sagittal images (Figure 3b).
- Open the slice view on the first tri-plane localizer images.
- 31P spectroscopy sequence:
- Use the following non-localized pulse-acquire sequence parameters: TR: 1,000 msec; TE: 0.34 msec; spectral width: 2,000 Hz; flip angle: 90 degrees; acquired data points: 1,024; 4 averages resulting in a time resolution of 1 spectrum every 6 sec.
- 31P shim box placement:
- Using a mouse, drag the second triplane localizer images into the viewing window at the top of the screen. Drag the spectroscopy sequence into the protocol window and double-click to open.
- Use the position tool bar to visualize the shim voxel (select the black rectangle with horizontal lines). After selecting this option, observe a green box on the localizer images.
NOTE: This is the shim voxel. - Move the voxel by left-clicking and holding the voxel in the center. Change the size and rotate the orientation of the voxel by left-clicking and holding the voxel at the corner of the box. Place the shim box so as to ensure B0 field homogeneity directly below the coil and parallel to the plane of the quadriceps.
NOTE: This is to ensure proper shimming within the sensitive region under the coil, which is the volume of tissue directly below the center of the coil. - Use the tri-plane localizer images to identify the sensitive region of the coil and adjust the shim box to encompass this region within the quadriceps muscle.
NOTE: The shim box can be larger than the true coverage of the surface coil in order to ensure B0 homogeneity within the data acquisition voxel (Figure 3c). - 31P test acquisition:
- Open the acquisition viewer window and select the head icon in the acquisition tool bar. This will allow for viewing of the spectroscopy acquisition in real time.
- After placement of the 31P shim voxel, run the sequence to obtain a single spectrum by clicking the "run" button at the top of the protocol window.
- Examine the quality of B0 shimming. Observe the resulting spectrum in the acquisition window. Observe a prominent PCr peak centered at 0 ppm and no significant noise (Figure 4a, left).
NOTE: Troubleshooting: If the spectrum appears noisy, ensure that the shim box is placed within muscle. Adjust the size and position of the shim box to improve the signal-to-noise ratio. Repeat the test acquisition as needed. - In order to see the PCr peak height, open the spectrum in the spectroscopy tool ("Applications" → "Spectroscopy"). Open the patient's folder (folder tree icon), select the appropriate scan, and double-click to load the spectrum.
- Pre-exercise T1 image:
- Obtain a single-slice axial T1-weighted image at the center of a coil.
- 31P pre-exercise acquisition:
- Copy the sequence from step 4.4 (that produced the best spectral quality) by left-clicking and dragging the sequence in the protocol window. Use this sequence for all subsequent measurements.
- In the sequence routine window, increase the number of measurements from 1 to 10. Select run to acquire 10 measurements while the subject is at rest.
- 31P exercise acquisition:
NOTE: Make careful note of the start and end exercise times, as this will be important for analysis.- Rest: Apply the shim settings from the previous scan and set the sequence to acquire 20 measurements. Instruct the subject to begin kicking after a countdown. Instruct the subject to remain at rest for 2 measurements.
- Exercise: Ask the subject to perform the knee extension exercise for ~ 30 sec (or the time required to achieve a 30% decrease in the PCr peak amplitude). After the subject achieves sufficient PCr depletion, ask them to rest.
- 31P post-exercise acquisitions:
- Acquire an additional 20 measurements at rest. Ensure that the post-exercise acquisitions begin immediately following the exercise sequence, without pause or shimming (Figure 4a, right).
NOTE: The subdivision of this recovery period into two separate acquisitions permits the analysis of the initial 20 dynamic spectra during the acquisition of the second 20 dynamic spectra, allowing the operator to avoid acquisition of the full recovery period if the exercise needs to be repeated.
- Acquire an additional 20 measurements at rest. Ensure that the post-exercise acquisitions begin immediately following the exercise sequence, without pause or shimming (Figure 4a, right).
- Ensuring exercise quality:
- Compare the PCr peak heights at the beginning and end of exercise. High-quality exercise sessions result in a ~ 30% decrease in the PCr concentration.
- Verify that the PCr peak height is the same at beginning of rest and at the end of recovery (typically, < 10% difference is desired). This ensures that there was negligible loss of field homogeneity during acquisition.
NOTE: If the PCr breakdown is insufficient, or if there has been a loss of field homogeneity, then repeat the exercise/recovery portion of the exam (taking care to avoid fatigue), ensure that the coil and straps are securely attached, and extend the duration of exercise and/or encourage more vigorous exercise (Figure 4b).
NOTE: A comparison of the images obtained in steps 6 and 11 permits an additional quality control step to visualize any displacement of the thigh and coil due to the exercise, thus ensuring that minimal motion occurred during the protocol, which could significantly affect the acquired data.
- Following post-exercise T1 imaging, repeat the pre-exercise axial T1 imaging (step 4.5) using the same acquisition parameters.
- In addition to sufficient depletion of PCr, measure the end exercise pH to ensure that the exercise did not induce acidosis of the muscle.
- Perform this by measuring the chemical shift between Pi and PCr (δPi) and using the following equation60:
pH = 6.77 + log[(δPi-3.29)/(5.68-δPi)]
NOTE: The pH should remain greater than 6.861. If the PCr breakdown is sufficient but the pH is too low, repeat the exercise bout for a shorter duration and/or with a decreased intensity.
- Saving Data:
- Save all acquired spectra as DICOM files and export them for processing using JMRUI.
- If using a scanner, select all spectroscopy acquisitions in the "Navigator" window.
- Under "Applications," select "Dicom Tools" → "Export MR Spectroscopy," and save the DICOM (*.dcm) files to C:/User/MedCom/temp/CDROFFLINE
(the tool automatically chooses this location). - Under "Transfer," select "Export to Offline." Save to the desired location.
5. Data Processing and Analysis62
- Analyze the MR spectra with freely-available JMRUI software (version 5.2; http://www.jmrui.eu/).
- Apodize and phase shift the spectra to ensure uniformity over all acquired time points (Figure 5). The PCr peak will be centered at 0 ppm in the spectra.
- Use the built-in AMARES algorithm to quantify the amplitude of the PCr peak in each acquired spectrum. The peak amplitude represents the concentration of PCr within the sensitive region of the surface coil at that particular time point.
- In the computational software, plot the PCr concentrations as a function of acquisition time. Using the built-in computational software curve-fit tool, fit the PCr recovery period data to the following equation52,63:
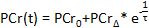
- Record the values of the baseline PCr (
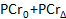 ), the lowest PCr (
), the lowest PCr ( ), and the recovery time(
), and the recovery time(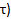 .
. - Ensure that the appropriate conditions are met during the exercise session by calculating the PCr depletion, the percent difference between the baseline PCr and the lowest PCr. Ideal exercise sessions result in a 20 - 50% depletion.
NOTE: The quality of the curve fitting can be ensured by verifying that the R2 value is greater than 0.75. R2 values are automatically calculated by the fitting software.
Results
Reproducibility Study
Six volunteers (4 men and 2 women; mean age: 24.5 ± 6.2 years) with no self-reported heart, metabolic, or mitochondrial disease underwent sessions of the described 31PMRS exercise and imaging technique on 2 different days within 1 week to evaluate technique reproducibility (Figure 6a). The studies performed on normal volunteers confirm the reproducibility of...
Discussion
This paper describes a standard protocol for 31PMRS examination that affords serial and noninvasive in vivo measurement of skeletal muscle mitochondrial function. The protocol holds considerable appeal when considering the breadth of investigations targeting the growing burden of metabolic syndrome and its resulting morbidity and mortality. This 31PMRS protocol requires a minimal amount of scanner time and can be incorporated into comprehensive metabolic investigations in subjects at any ce...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported in part by a Davis Heart and Lung Research Institute Trifit Award, as well as by the Intramural Research Program of the NIH National Institute on Aging.
Materials
| Name | Company | Catalog Number | Comments |
| 1.5 T MR Scanner | Siemens | manufacturer will not affect results | |
| 10 cm 31P transmit-receive coil, 1.5T compatible | PulseTeq | manufacturer will not affect results | |
| 3 fl oz Baby Oil | Johnson & Johnson | manufacturer will not affect results | |
| Foam triangle cushion (Knee) | Siemens | manufacturer will not affect results | |
| (3) plastic buckle resistive straps; table to table | Siemens | manufacturer will not affect results | |
| (1) plastic buckle resistive strap; self-connecting | Siemens |
References
- Eckel, R. H., Alberti, K. G., Grundy, S. M., Zimmet, P. Z. The metabolic syndrome. Lancet. 375 (9710), 181-183 (2010).
- Shulman, G. I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 371 (12), 1131-1141 (2014).
- Holmstrom, M. H., Iglesias-Gutierrez, E., Zierath, J. R., Garcia-Roves, P. M. Tissue-specific control of mitochondrial respiration in obesity-related insulin resistance and diabetes. Am J Physiol Endocrinol Metab. 302 (6), 731-739 (2012).
- Jheng, H. F., et al. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol. 32 (2), 309-319 (2012).
- Petersen, K. F., et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 300 (5622), 1140-1142 (2003).
- Kelley, D. E., He, J., Menshikova, E. V., Ritov, V. B. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 51 (10), 2944-2950 (2002).
- Liu, R., et al. Impaired mitochondrial dynamics and bioenergetics in diabetic skeletal muscle. PLoS One. 9 (3), 92810 (2014).
- Ha, H., Hwang, I. A., Park, J. H., Lee, H. B. Role of reactive oxygen species in the pathogenesis of diabetic nephropathy. Diabetes Res Clin Pract. 82, 42-45 (2008).
- Akude, E., et al. Diminished superoxide generation is associated with respiratory chain dysfunction and changes in the mitochondrial proteome of sensory neurons from diabetic rats. Diabetes. 60 (1), 288-297 (2011).
- Fernyhough, P. Mitochondrial dysfunction in diabetic neuropathy: a series of unfortunate metabolic events. Curr Diab Rep. 15 (11), 89 (2015).
- Chen, M., Wang, W., Ma, J., Ye, P., Wang, K. High glucose induces mitochondrial dysfunction and apoptosis in human retinal pigment epithelium cells via promoting SOCS1 and Fas/FasL signaling. Cytokine. 78, 94-102 (2016).
- Blake, R., Trounce, I. A. Mitochondrial dysfunction and complications associated with diabetes. Biochim Biophys Acta. 1840 (4), 1404-1412 (2014).
- Rains, J. L., Jain, S. K. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 50 (5), 567-575 (2011).
- Serviddio, G., et al. Mitochondrial involvement in non-alcoholic steatohepatitis. Mol Aspects Med. 29 (1-2), 22-35 (2008).
- Perez-Carreras, M., et al. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology. 38 (4), 999-1007 (2003).
- Garcia-Ruiz, I., et al. Mitochondrial complex I subunits are decreased in murine nonalcoholic fatty liver disease: implication of peroxynitrite. J Proteome Res. 9 (5), 2450-2459 (2010).
- Patti, M. E., Corvera, S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr Rev. 31 (3), 364-395 (2010).
- Muoio, D. M., Newgard, C. B. Obesity-related derangements in metabolic regulation. Annu Rev Biochem. 75, 367-401 (2006).
- Bonnard, C., et al. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest. 118 (2), 789-800 (2008).
- Jheng, H. F., Huang, S. H., Kuo, H. M., Hughes, M. W., Tsai, Y. S. Molecular insight and pharmacological approaches targeting mitochondrial dynamics in skeletal muscle during obesity. Ann N Y Acad Sci. 1350, 82-94 (2015).
- Coen, P. M., Goodpaster, B. H. Role of intramyocelluar lipids in human health. Trends Endocrinol Metab. 23 (8), 391-398 (2012).
- Montgomery, M. K., Turner, N. Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect. 4 (1), 1-15 (2015).
- Martelli, A., Puccio, H. Dysregulation of cellular iron metabolism in Friedreich ataxia: from primary iron-sulfur cluster deficit to mitochondrial iron accumulation. Front Pharmacol. 5, 130 (2014).
- Campuzano, V., et al. Frataxin is reduced in Friedreich ataxia patients and is associated with mitochondrial membranes. Hum Mol Genet. 6 (11), 1771-1780 (1997).
- Calabrese, V., et al. Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich's ataxia. J Neurol Sci. 233 (1-2), 145-162 (2005).
- Ristow, M., et al. Frataxin activates mitochondrial energy conversion and oxidative phosphorylation. Proc Natl Acad Sci U S A. 97 (22), 12239-12243 (2000).
- Ardehali, H., et al. Targeting myocardial substrate metabolism in heart failure: potential for new therapies. Eur J Heart Fail. 14 (2), 120-129 (2012).
- Ren, J., Pulakat, L., Whaley-Connell, A., Sowers, J. R. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med (Berl). 88 (10), 993-1001 (2010).
- Marin-Garcia, J., Goldenthal, M. J. Understanding the impact of mitochondrial defects in cardiovascular disease: a review. J Card Fail. 8 (5), 347-361 (2002).
- Babcock, M., et al. Regulation of mitochondrial iron accumulation by Yfh1p, a putative homolog of frataxin. Science. 276 (5319), 1709-1712 (1997).
- Foury, F., Cazzalini, O. Deletion of the yeast homologue of the human gene associated with Friedreich's ataxia elicits iron accumulation in mitochondria. FEBS Lett. 411 (2-3), 373-377 (1997).
- Wardman, P., Candeias, L. P. Fenton chemistry: an introduction. Radiat Res. 145 (5), 523-531 (1996).
- Lodi, R., et al. Cardiac energetics are abnormal in Friedreich ataxia patients in the absence of cardiac dysfunction and hypertrophy: an in vivo 31P magnetic resonance spectroscopy study. Cardiovasc Res. 52 (1), 111-119 (2001).
- Raman, S. V., et al. Impaired myocardial perfusion reserve and fibrosis in Friedreich ataxia: a mitochondrial cardiomyopathy with metabolic syndrome. Eur Heart J. 32 (5), 561-567 (2011).
- Kitzman, D. W., et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 306 (9), 1364-1370 (2014).
- Scheuermann-Freestone, M., et al. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation. 107 (24), 3040-3046 (2003).
- Allcock, D. M., Sowers, J. R. Best strategies for hypertension management in type 2 diabetes and obesity. Curr Diab Rep. 10 (2), 139-144 (2010).
- Katzmarzyk, P. T., Church, T. S., Janssen, I., Ross, R., Blair, S. N. Metabolic syndrome, obesity, and mortality: impact of cardiorespiratory fitness. Diabetes Care. 28 (2), 391-397 (2005).
- Wang, J., et al. The metabolic syndrome predicts cardiovascular mortality: a 13-year follow-up study in elderly non-diabetic Finns. Eur Heart J. 28 (7), 857-864 (2007).
- Zambon, S., et al. Metabolic syndrome and all-cause and cardiovascular mortality in an Italian elderly population: the Progetto Veneto Anziani (Pro.V.A) Study. Diabetes Care. 32 (1), 153-159 (2009).
- Malik, S., et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 110 (10), 1245-1250 (2004).
- Ropper, A. H., Samuels, M. A. . Adams and Victor's Principles of Neurology. 9 edn. , (2009).
- Abeti, R., et al. Targeting lipid peroxidation and mitochondrial imbalance in Friedreich's ataxia. Pharmacol Res. 99, 344-350 (2015).
- Li, Y., et al. Excision of Expanded GAA Repeats Alleviates the Molecular Phenotype of Friedreich's Ataxia. Mol Ther. 23 (6), 1055-1065 (2015).
- Toledo, F. G., Goodpaster, B. H. The role of weight loss and exercise in correcting skeletal muscle mitochondrial abnormalities in obesity, diabetes and aging. Mol Cell Endocrinol. 379 (1-2), 30-34 (2013).
- Oldridge, N. B., Guyatt, G. H., Fischer, M. E., Rimm, A. A. Cardiac rehabilitation after myocardial infarction. Combined experience of randomized clinical trials. JAMA. 260 (7), 945-950 (1988).
- O'Connor, G. T., et al. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation. 80 (2), 234-244 (1989).
- Ryan, T. E., Brizendine, J. T., McCully, K. K. A comparison of exercise type and intensity on the noninvasive assessment of skeletal muscle mitochondrial function using near-infrared spectroscopy. J Appl Physiol (1985). 114 (2), 230-237 (2013).
- Wallimann, T. Bioenergetics. Dissecting the role of creatine kinase. Curr Biol. 4 (1), 42-46 (1994).
- Forbes, S. C., Paganini, A. T., Slade, J. M., Towse, T. F., Meyer, R. A. Phosphocreatine recovery kinetics following low- and high-intensity exercise in human triceps surae and rat posterior hindlimb muscles. Am J Physiol Regul Integr Comp Physiol. 296 (1), 161-170 (2009).
- Korzeniewski, B., Rossiter, H. B. Each-step activation of oxidative phosphorylation is necessary to explain muscle metabolic kinetic responses to exercise and recovery in humans. J Physiol. 593 (24), 5255-5268 (2015).
- Meyer, R. A. A linear model of muscle respiration explains monoexponential phosphocreatine changes. Am J Physiol. 254 (4), 548-553 (1988).
- McCully, K. K., Fielding, R. A., Evans, W. J., Leigh, J. S., Posner, J. D. Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol (1985). 75 (2), 813-819 (1993).
- Layec, G., Haseler, L. J., Richardson, R. S. Reduced muscle oxidative capacity is independent of O2 availability in elderly people. Age (Dordr). 35 (4), 1183-1192 (2013).
- Larson-Meyer, D. E., Newcomer, B. R., Hunter, G. R., Hetherington, H. P., Weinsier, R. L. 31P MRS measurement of mitochondrial function in skeletal muscle: reliability, force-level sensitivity and relation to whole body maximal oxygen uptake. NMR Biomed. 13 (1), 14-27 (2000).
- Kemp, G. J., Ahmad, R. E., Nicolay, K., Prompers, J. J. Quantification of skeletal muscle mitochondrial function by 31P magnetic resonance spectroscopy techniques: a quantitative review. Acta Physiol (Oxf). 213 (1), 107-144 (2015).
- Lynch, D. R., et al. Near infrared muscle spectroscopy in patients with Friedreich's ataxia. Muscle Nerve. 25 (5), 664-673 (2002).
- Nachbauer, W., et al. Bioenergetics of the calf muscle in Friedreich ataxia patients measured by 31P-MRS before and after treatment with recombinant human erythropoietin. PLoS One. 8 (7), 69229 (2013).
- Kanal, E., et al. ACR guidance document on MR safe practices: 2013. J Magn Reson Imaging. 37 (3), 501-530 (2013).
- Petroff, O. A., Ogino, T., Alger, J. R. High-resolution proton magnetic resonance spectroscopy of rabbit brain: regional metabolite levels and postmortem changes. J Neurochem. 51 (1), 163-171 (1988).
- Jubrias, S. A., Crowther, G. J., Shankland, E. G., Gronka, R. K., Conley, K. E. Acidosis inhibits oxidative phosphorylation in contracting human skeletal muscle in vivo. J Physiol. 553 (2), 589-599 (2003).
- Layec, G., et al. Reproducibility assessment of metabolic variables characterizing muscle energetics in vivo: A 31P-MRS study. Magn Reson Med. 62 (4), 840-854 (2009).
- Iotti, S., Lodi, R., Frassineti, C., Zaniol, P., Barbiroli, B. In vivo assessment of mitochondrial functionality in human gastrocnemius muscle by 31P MRS. The role of pH in the evaluation of phosphocreatine and inorganic phosphate recoveries from exercise. NMR Biomed. 6 (4), 248-253 (1993).
- Wren, T. A., Bluml, S., Tseng-Ong, L., Gilsanz, V. Three-point technique of fat quantification of muscle tissue as a marker of disease progression in Duchenne muscular dystrophy: preliminary study. AJR Am J Roentgenol. 190 (1), 8-12 (2008).
- Milani, R. V., Lavie, C. J., Mehra, M. R., Ventura, H. O. Understanding the basics of cardiopulmonary exercise testing. Mayo Clin Proc. 81 (12), 1603-1611 (2006).
- Wust, R. C., van der Laarse, W. J., Rossiter, H. B. On-off asymmetries in oxygen consumption kinetics of single Xenopus laevis skeletal muscle fibres suggest higher-order control. J Physiol. 591 (3), 731-744 (2013).
- Ryan, T. E., Brophy, P., Lin, C. T., Hickner, R. C., Neufer, P. D. Assessment of in vivo skeletal muscle mitochondrial respiratory capacity in humans by near-infrared spectroscopy: a comparison with in situ measurements. J Physiol. 592 (15), 3231-3241 (2014).
- Hamaoka, T., McCully, K. K., Niwayama, M., Chance, B. The use of muscle near-infrared spectroscopy in sport, health and medical sciences: recent developments. Philos Trans A Math Phys Eng Sci. 369, 4591-4604 (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved