A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Scanning Electron Microscopy (SEM) Protocols for Problematic Plant, Oomycete, and Fungal Samples
In This Article
Summary
Problems in the processing of biological samples for scanning electron microscopy observation include cell collapse, treatment of samples from wet microenvironments and cell destruction. Low-cost and relatively rapid protocols suited for preparing challenging samples such as floral meristems, oomycete cysts, and fungi (Agaricales) are compiled and detailed here.
Abstract
Common problems in the processing of biological samples for observations with the scanning electron microscope (SEM) include cell collapse, treatment of samples from wet microenvironments and cell destruction. Using young floral tissues, oomycete cysts, and fungi spores (Agaricales) as examples, specific protocols to process delicate samples are described here that overcome some of the main challenges in sample treatment for image capture under the SEM.
Floral meristems fixed with FAA (Formalin-Acetic-Alcohol) and processed with the Critical Point Dryer (CPD) did not display collapsed cellular walls or distorted organs. These results are crucial for the reconstruction of floral development. A similar CPD-based treatment of samples from wet microenvironments, such as the glutaraldehyde-fixed oomycete cysts, is optimal to test the differential growth of diagnostic characteristics (e.g., the cyst spines) on different types of substrates. Destruction of nurse cells attached to fungi spores was avoided after rehydration, dehydration, and the CPD treatment, an important step for further functional studies of these cells.
The protocols detailed here represent low-cost and rapid alternatives for the acquisition of good-quality images to reconstruct growth processes and to study diagnostic characteristics.
Introduction
In biology, the use of scanning electron microscopy (SEM) has been extended to studies of structural evolution, comparative morphology, organ development, and characterization of populations or species1. With its two-dimensional view of microscopic structures, areas such as micromorphology and systematics profited from SEM technique advances since the second half of the 20th century. For example, the introduction of the sputter coating methodology in the 1970s made possible observations of delicate materials such as shoot apices and flowers enhancing the imaging of non-conductive tissues2,3. SEM uses electrons ejected from the surface of the specimen to reproduce the topography in a high-vacuum environment4.
Studies involving SEM are focused in both the inference of structural characters and the reconstruction of growth processes. New structural characters relevant to the taxonomy and systematics of a wide range of organisms have been discovered from SEM observations. For example, plant traits used for species diagnosis or supraspecific classifications, such as the vestured pits of wood5, stigma diversity6, nectary and floral morphology7,8, trichome details9, and pollen grains10,11, cannot be properly visualized without SEM. Successful observations with conventional SEM have been also achieved for long-time formalin-fixed organisms12 and plant herbarium specimens13.
On the other hand, studies of reconstruction of growth processes using SEM involve a wide range of topics, such as organ development14, infections induced by bacteria15, plant root physiology16, parasite-host attachment mechanisms17,18, drug effects on parasites19, mycoparasitism and antibiosis20,21, growth malformation22, comparative development of wild and mutant individuals23, and entire life cycles24. Although environmental scanning electron microscopes (ESEM)25 may have important advantages for the observation of wet biological samples in growth processes, delicate material may still be compromised even in the low vacuum condition of the ESEM), and need to be processed adequately to avoid loss of valuable morphological observation.
In this paper, a review of specific protocols for SEM observation of three different types of samples is presented: floral meristems, oomycetes (Saprolegnia), and fungal material. These protocols compile the experience of our previous SEM-based studies26,27,28,29,30,31,32,33, where specific difficulties and alternative solutions have been found. In the case of plant comparative developmental and structural studies, the use of SEM started in the 1970s34,35, and since then, researchers discovered that certain floral features are more labile than previously thought36. Reconstruction of floral development involves the capture of all stages between young floral meristems and anthesis. To reach this aim, it is essential that the sample topography and the cell wall integrity are not compromised after the fixation and subsequent dehydration. Young floral meristems are particularly vulnerable to cell wall collapse (Figures 1a, 1b). Similarly, delicate structures such as nectaries, petals, stigmas and sporangia require effective and undamaging protocols. This review summarizes an optimal protocol to keep young and delicate tissues intact for SEM imaging.
In the case of the oomycetes (Stramenopiles)-one of the most diverse and widespread groups of parasites, with hosts ranging from microbes and plants to invertebrates and vertebrates37- there are spores that grow and develop in a wet environment. This condition represents a challenge for SEM observation because the spores need an adequate substrate not suitable for standard SEM protocols. Among the oomycetes, species of Saprolegnia are of particular interest because they can cause severe reductions in aquacultures, fisheries, and amphibian populations38. Micromorphological characteristics, such as the hooked spines of cysts, have been found to be useful to identify species of Saprolegnia, which is fundamental to establish infection controls and potential treatments39. Here, there is an experimental protocol to compare the patterns of the spine growth of cysts on different substrates and to manipulate the sample for critical point dryer (CPD) preparation and subsequent SEM observation.
In a third case, there are interesting findings that came up after an inspection of the spores of the fungi Phellorinia herculanea f. stellata f. nova (Agaricales)31. Together with the spores, a group of unexpected nursery cells was identified under the SEM. With previous traditional protocols and untreated material, the nurse cells came out completely collapsed (Figure 1c). Further inferences about particular tissues associated to the spores can be made with the simple but crucial modifications to the standard approaches described here (Figure 1d).
In this review, there are detailed SEM protocols that can be used to deal with different problems associated with SEM observation in angiosperms, oomycetes, and Agaricales, such as cell collapse and meristematic tissue shrinking, non-optimal growth of cyst spines, and destruction of ephemeral tissues, respectively.

Figure 1: Comparison of samples treated without (a, c) and with (b, d) the protocol FAA-ethanol-CPD. (a-b) Floral buds of Anacyclus clavatus, mid-development. Bud treated with osmium tetroxide46 (a) and bud treated with the FAA-CPD protocol (b). (c-d) Nurse cells with spores of Phellorinia herculanea f. stellata. Dried samples without any treatment (c) and with the protocol here described for Agaricales (d). Spores in orange. Scales: (a-b) 100 µm, (c-d) 50 µm. Photos were taken by Y. Ruiz-León. Please click here to view a larger version of this figure.
Protocol
NOTE: This protocol includes six main sections, three devoted to specific organisms (sections 1-3), and three describing the procedures common to all (4-6). Asterisks (*) indicate steps modified by the experimenters.
1. Studies of Developing and Fully Formed Plant Structures
- Collection and fixation
- If the plant material is collected in a place with no access to a fume cupboard, introduce and immerse the material in 70% ethanol in centrifuge tubes. Ideally, immerse the material after 48 h in FAA (steps 1.1.1-1.1.3) to avoid excessive dehydration in the ethanol. If a fume cupboard is accessible to the plant material, ignore this step and continue with 1.1.1.
- Prepare the formalin-acetic-alcohol (FAA) fixative in a fume cupboard fitted with an aldehyde filter. Add 85 parts of 70% denatured ethanol, 10 parts of 60% formaldehyde solution, and 5 parts of glacial acetic acid. Prepare the FAA just before fixing the material, as its long term storage is not recommended40.
- Under the fume cupboard, pour the stock of FAA into individual wide-mouth and leak-proof plastic bottles. Use as many bottles as there are samples available, and create labels for sample identification.
- Select the floral or vegetative meristems to fix, ensuring that they are not damaged by insects, fungi, or extreme weather conditions. Cut the branches, removing unwanted material, and deposit the sample immediately in the FAA solution.
- After 72-96 h, pour the FAA into a plastic container for chemical disposal. Immediately, wash the samples three times with fresh 70% ethanol to remove any residual FAA. Fixed material can be stored indefinitely in 70% ethanol.
- Dissection and dehydration
- Dissect the fixed material in 70% ethanol under the stereomicroscope using ultra fine tweezers, needles, forceps, brushes, and micro-scalpels (the maximum size of the tissue should be around 1 cm3, or 2 cm for flat material). Dissect the samples into a Petri dish covered with ethanol to prevent the tissues from drying. Use a Petri dish with the base covered with dry black silicon to better see the contrasting white tissues.
- Put the dissected material in specimen containers for the critical point dryer (CPD, Figure 2a). At this point, immerse the containers into the Petri dish with 70% ethanol, and include the sample identification labels (made with paper and pencil). For more effective drying for further manipulation, avoid mixing the young and mature samples in the same container.*
- Put the lids on the containers and deposit them in plastic centrifuge tubes with plenty of 70% ethanol. Store the tubes overnight if the material is not processed immediately.
- Transfer the dissected material through the following ethanol series in hermetic jars or centrifuge tubes: 70%, 90%, 100%, and 100%. Leave the samples in each solution for 1 h at least. Keep the samples overnight in a 100% ethanol solution.
- Transfer the containers with the material to the CPD (section 4).
- Mounting and preparing plant tissues for SEM observation
- Write the sample identification number underneath the SEM sample holders (i.e., aluminum stubs). Cover the top of the stubs with double-sided tape. Place the stubs into a specimen holder (Figure 2b).
- Under a stereomicroscope, carefully open the containers carrying the young and delicate samples already dried in the CPD. Bear in mind that after the CPD treatment, the samples become lighter and sensitive to electrostatics. Close the containers once the samples have been taken out to avoid dust or impurities.
- Put the samples on the sticky surface of the stubs, planning ahead the desired position (once the samples touch the surface, it is very difficult to remove them). Do not try to carry a major dissection at this point; just remove unwanted tissue that is easy to pick up. For palynological studies, dissect the anthers and open them to expose the pollen on the stubs.
- Put long samples (e.g., 2 cm long) such as inflorescences in the horizontal position. When possible, orient samples of the same structure for polar, side, and bottom views. Leave enough space between samples on the stub.
- If the samples cannot be processed immediately, keep them protected overnight in a hermetic container with silica gel to avoid rehydration (Figure 2c)*. Coat the samples using the sputter coater and transfer them to the SEM (sections 5 and 6).
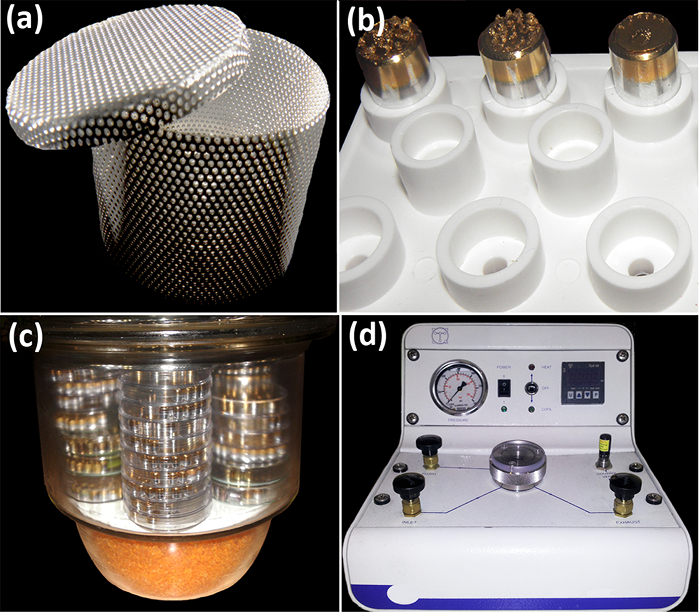
Figure 2: Tools for sample manipulation and processing before SEM observation. (a) Steel-made specimen container with holed walls for the ethanol/CO2 interchange in the CPD chamber. (b) Steel stubs within a plastic specimen holder. (c) Glass container used to keep the samples protected from humidity and dust. At the base, there is a compartment for silica gel. (d) Critical Point Dryer. In the front, there are (from left to right) the manometer, the power switch, the temperature control system, and the temperature display. Usual working pressure for CO2-ethanol interchange is 60 bars (800 psi). In the top, there are four valves (inlet, drain, ventilation, and exhaust controls) flanking the central sample chamber. Photos were taken by Y. Ruiz-León and M.A. Bello. Please click here to view a larger version of this figure.
2. Study of Cyst Behavior of Saprolegnia (Oomycetes) on Different Surfaces
- Growing and fixing the cysts
- Prepare peptone and glucose (PG-l) media41 using D-(+)-glucose (6 g) and mycological peptone (3 g)*. Add up to 900 mL of tap water and autoclave 40 min at 121 °C. Pour 50 mL of the previously autoclaved solution A (NaH2PO4, 0.13 M) and 50 mL of solution B (Na2HPO4, 0.13 M).
- From stock cultures of strains of Saprolegnia parasitica maintained on peptone, glucose, agar media (PGA, which is prepared as PG-l but adding 10 g of European bacteriological agar to the glucose and the peptone before autoclave), grow mycelia colonies in 0.5 mL of PG-l droplets for 24-48 h at 20 °C in Petri dishes. Induce sporulation by washing the mycelia with autoclaved tap water three times and incubating them for 15 h at 20 °C42,43.
- Collect the released secondary zoospores by gently pipetting the upper part of the suspension and pool them in 1 mL portions. Agitate vigorously the zoospores for 30 s by vortexing to produce secondary cysts44.
- To test the differential growth of the spines of the cysts, on separate Petri dishes (p60), put 0.5 mL of the secondary cyst suspension onto different surfaces (i.e., carbon, gold, and copper TEM grids; salmon and hake fish scales (previously bleached); and glass cover slips)*. Incubate the cysts at 20 °C for 70 min, which favors the attachment of the cysts to the surface.
- Remove the liquid and add 0.5 mL of 2% glutaraldehyde to each surface for the fixation of the cysts. Keep the samples at room temperature under a fume cupboard for 1 h.
- Remove the glutaraldehyde and dehydrate the sample through an ethanol series (30%, 40%, 50%, 60%, 70%, 80%, 90%, 100%, and 100%), adding 5 mL of each ethanol solution for 15 min. Once in the last 100% ethanol solution, the sample can be stored for up to a month in a sealed Petri dish. At this stage, the samples are ready to be dried in the CPD.
- Carefully transfer the grids and the scales from the Petri dish to a holder suitable for the CPD (Section 4). For this step, use a CPD grid holder or a stacking specimen holder, which keep samples separated from each other. Take the grids and scales with tweezers, keeping in mind that the cysts should be facing up on the grids all the time.*
- Mounting and preparing cyst samples for SEM observation
- Mount the grids and the scales on the aluminum stubs that were previously covered with double-sided carbon tape and labelled underneath.
- Transfer the samples to the sputter coater (section 5).
- Observe the samples under the SEM (section 6).
3. Study of Herbarium Fungal Spores of Phellorinia herculanea under SEM
- Rehydration and dehydration of spores
- Wrap each sample carefully with filter paper, forming pencil-labeled envelopes ~0.5-1 cm2, taking care not to crush them. Seal the filter paper with paper clips. Transfer the packed samples to a Petri dish and immerse them in 10 mL of water to rehydrate tissues around the spores.
- Immediately put the samples in a microwave (600 W for approximately 20 s). Remove the material once the water starts to evaporate, and allow it to cool down at room temperature.
- Pass the samples through the following ethanol series: 30%, 50%, 70%, 80%, 90%, 95%, 100%, and 100%. Depending on the amount of samples, use a beaker or centrifuge tubes for this step. Leave the samples for 15 min in each solution.
- Place the samples to the CPD (section 4).
- Mounting and preparing spores for SEM observation
- Open the envelopes. Pour the spores on a previously-prepared stub with double-sided tape. Alternatively, collect the spores with the sticky surface of the stubs, taking care not to crush them*.
- If the samples contain few spores, in addition to the previous step, cut a small piece of the envelope (~1 mm2) and place it on a new stub*.
- Place the tissues into the sputter coater (section 5).
- Observe under the SEM (section 6).
4. Drying of Material Using a Critical Point Dryer (CPD, Figure 2d)
- Use the CPD in a ventilated area and verify that all the valves of the machine are closed. Check if the sample chamber is empty and clean.
- Switch on the machine and verify that the temperature control system test takes place automatically. If the CPD has an external refrigeration bath system, check the water levels before switching it on.
- Follow the manufacturer's instructions of the specific CPD used for the ethanol and CO2 interchange. For safety, carry on this step under the supervision of someone trained for the use of the machine. Remember that it is exposed to rapid pressure changes, it could blow out violently.
- Take out the samples and continue with the step 1.3 if working with plant tissues, step 2.2 if working with oomycetes cysts, and steps 3.2 if working with fungi spores.
5. Coating the Samples with Gold Using the Sputter Coater (Figure 3a)
- Check the sputter coater. Verify that the gold cathode target is in good condition. Use a lint-free cloth drenched with 90% ethanol to clean the walls of the vacuum chamber and the chamber lid if necessary.
- Mark the sputter holder with numbers beside each stub hole for further identification of the samples under the microscope. Carefully, place the stubs loaded with the samples and secure them. Use a rotary planetary specimen stage to ensure a uniform coating on specimens with irregular surfaces.
- Follow the manufacturer's instructions to adjust settings such as the working distance (e.g., 30 mm), operation gas pressure (e.g., 5 x 10-1 - 7 x 10-1 mbar), the sputtering time (e.g., 50 s), thickness of the gold layer (e.g., 12 nm) the current (e.g., 15 mA) and the voltage supply (e.g., 600 V)45.
- Remove the stubs and take them to the SEM (section 6). Alternatively, place the stubs into a sealed container with silica gel (Figure 2c).
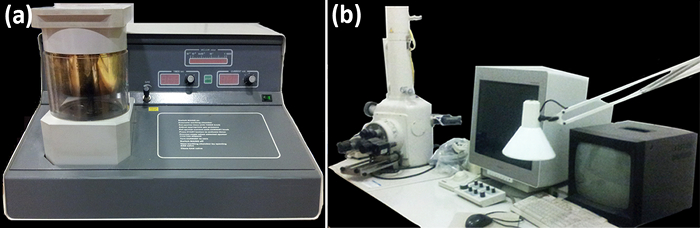
Figure 3: Sputter coater (a) and scanning electron microscope (b). (a) Front view of the vacuum chamber (left), gas valve, timer, vacuum, and current controls. (b) Side view of the SEM main components (from left to right): the vacuum column with the sample chamber, the computer screen with the controls, and the chamber's monitor. Photos were taken by Y. Ruiz-León. Please click here to view a larger version of this figure.
6. Observation under the Scanning Electron Microscope (SEM, Figure 3b)
- SEM start up
- Follow the manufacturer's instructions to start and set the SEM, adjusting the sample height the objective aperture diameter (e.g., for plants 2 µm and for fungi and oomycetes 4 µm), the operating voltage (e.g., 15 kV).
- Check the correct alignment of the electron beam system and set the axial alignment and the stigmators according to the manufacturers indications. Adjust the working distance in order to obtain an adequate depth of field.
- Image capture
- Get a focused image of the sample and use it as a starting point. Increase the magnification close to the maximum level and focus the image again. Choose areas with surface irregularities such as holes. Correct astigmatism and adjust the optimum contrast and brightness.
- Capture the SEM Image with the high-resolution. Use the BSE detector if the image shows that the samples are charged. Otherwise, set the SE detector. Change the detectors following the manufacturer's instructions.
Results
Floral Development and Fixation of Developing and Fully Formed Plant Structures
Using the FAA-CPD protocol described here, young and mature plant tissues are optimally fixed and dehydrated for SEM imaging. Processes such as floral development can be reconstructed because the topography and shape of the buds is not distorted by cell shrinking (Figures 1b, 1d, 4a-f). Structures with complex shapes can be successfull...
Discussion
With respect to standard SEM protocols, the procedures presented here include relatively rapid, easy to follow, and low-cost methodologies. Depending on the amount of samples and on the ease of processing, it takes four to five days to acquire good quality images. Including adequate safety precautions for the CPD and SEM operation, the procedures are easy to handle. Particular caution should be taken with formalin and the glutaraldehyde (see steps 1.1.1 to 1.1.3 and 2.1.5 of the protocol). There are certain steps where, ...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 634429. This publication reflects the views only of the author, and the European Commission cannot be held responsible for any use which may be made of the information contained therein. We also acknowledge the financial contribution made by the Real Jardín Botánico, CSIC. SR is grateful to the European Union [ITN-SAPRO-238550] for the support of her research in Saprolegnia. We also want to thank Francisco Calonge for kindly provide the Phellorinia herculanea images and B. Pueyo for processing samples (Figure 5). All images were taken by the SEM service at the Real Jardín Botánico-CSIC in Madrid.
Materials
| Name | Company | Catalog Number | Comments |
| Acetic acid | No specific supplier | Skin irritation, eye irritation | |
| aluminium stubs | Ted Pella, Inc. | 16221 | www.tedpella.com |
| Centrifuge tubes | No specific supplier | ||
| Critical Point Dryer | Polaron Quatum Technologies | CPD7501 | |
| D-(+)-Glucose | Merck | 1,083,421,000 | |
| Double sided sellotape | No specific supplier | ||
| Ethanol absolute | No specific supplier | Flammable | |
| European bacteriological agar | Conda | 1800.00 | www.condalab.com |
| Filter paper | No specific supplier | ||
| Forceps | No specific supplier | ||
| Formalin 4% | No specific supplier | Harmful, acute toxicity, skin sensitisation, carcinogenicity. Flammable | |
| Glass cover slips | No specific supplier | ||
| Glass hermetic container | No specific supplier | ||
| Glutaraldehyde 25% DC 253857.1611 (L) | Dismadel S.L. | 3336 | www.dismadel.com |
| Mycological peptone | Conda | 1922.00 | www.condalab.com |
| needles | No specific supplier | ||
| Petri dishes | No specific supplier | ||
| Plastic containers | No specific supplier | ||
| Sample holder with lid for the critical point dryer | Ted Pella, Inc. | 4591 | www.tedpella.com |
| scalpels | No specific supplier | ||
| Scanning Electron Microscope | Hitachi | S3000N | |
| Software for SEM | |||
| Solution A: NaH2PO4 | |||
| Solution B: Na2HPO4 | |||
| Specimen holders | No specific supplier | ||
| Sputter coater | Balzers | SCD 004 | |
| Stereomicroscope | No specific supplier | ||
| Transmission Electron Microscope (TEM) grids | Electron Microscopy Sciences | G200 (Square Mesh) | www.emsdiassum.com |
| Tweezers | No specific supplier |
References
- Endress, P. K., Baas, P., Gregory, M. Systematic plant morphology and anatomy: 50 years of progress. Taxon. 49 (3), 401-434 (2000).
- Falk, R. H., Gifford, E. M., Cutter, E. G. Scanning electron microscopy of developing plant organs. Science. 168 (3938), 1471-1474 (1970).
- Damblon, F. Sputtering, a new method of coating pollen grains in scanning electron microscopy. Grana. 15 (3), 137-144 (1975).
- Everhart, T. E., Thornley, R. F. M. Wide-band detector for micro-microampere low-energy electron currents. J. Sci. Instrum. 37 (7), 37246-37248 (1960).
- Collins, S. P., et al. Advantages of environmental scanning electron microscopy in studies of microorganisms. Microsc. Res. Techniq. 25 (5-6), 398-405 (1993).
- Fannes, W., Vanhove, M. P. M., Huyse, T., Paladini, G. A scanning electron microscope technique for studying the sclerites of Cichlidogyrus. Parasitol. Res. 114 (5), 2031-2034 (2015).
- Erbar, C., Leins, P. Portioned pollen release and the syndromes of secondary pollen presentation in the Campanulales-Asterales complex. Flora. 190 (4), 323-338 (1995).
- Jansen, S., Smets, E., Baas, P. Vestures in woody plants: a review. IAWA Journal. 19 (4), 347-382 (1998).
- Bortolin Costa, M. F., et al. Stigma diversity in tropical legumes with considerations on stigma classification. Bot. Rev. 80 (1), 1-29 (2014).
- Almeida, O. J. G., Cota-Sánchez, J. H., Paoli, A. A. S. The systematic significance of floral morphology, nectaries, and nectar concentration in epiphytic cacti of tribes Hylocereeae and Rhipsalideae (Cactaceae). Perspect. Plant Ecol. 15 (5), 255-268 (2013).
- Konarska, A. Comparison of the structure of floral nectaries in two Euonymus L. species (Celastraceae). Protoplasma. 252 (3), 901-910 (2015).
- Giuliani, C., Maleci Bini, L. Insight into the structure and chemistry of glandular trichomes of Labiatae, with emphasis on subfamily Lamioideae. Plant Syst. Evol. 276 (3-4), 199-208 (2008).
- Li, K., Zheng, B., Wang, Y., Zhou, L. L.Breeding system and pollination biology of Paeonia delavayi (Paeoniaceae), an endangered plant in the Southwest of China. Pak. J. Bot. 46 (5), 1631-1642 (2014).
- García, L., Rivero, M., Droppelmann, F. Descripción morfológica y viabilidad del polen de Nothofagus nervosa (Nothofagaceae). Bosque. 36 (3), 487-496 (2015).
- Prenner, G., Klitgaard, B. B. Towards unlocking the deep nodes of Leguminosae: floral development and morphology of the enigmatic Duparquetia orchidacea (Leguminosae, Caesalpinioideae). Am. J. Bot. 95 (11), 1349-1365 (2008).
- Ratnayake, K., Joyce, D. C., Webb, R. I. A convenient sample preparation protocol for scanning electron microscope examination of xylem-occluding bacterial biofilm on cut flowers and foliage. Sci. Hortic-Amsterdam. 140 (1), 12-18 (2012).
- Çolak, G., Celalettin Baykul, M., Gürler, R., Çatak, E., Caner, N. Investigation of the effects of aluminium stress on some macro and micro-nutrient contents of the seedlings of Lycopersicon esculentum Mill. by using scanning electron microscope. Pak. J. Bot. 46 (1), 147-160 (2014).
- Arafa, S. Z. Scanning electron microscope observations on the monogenean parasite Paraquadriacanthus nasalis from the nasal cavities of the freshwater fish Clarias gariepinus in Egypt with a note on some surface features of its microhabitat. Parasitol. Res. 110 (5), 1687-1693 (2012).
- Uppalapatia, S. R., Kerwinb, J. L., Fujitac, Y. Epifluorescence and scanning electron microscopy of host-pathogen interactions between Pythium porphyrae (Peronosporales, Oomycota)and Porphyra yezoensis (Bangiales, Rhodophyta). Bot. Mar. 44 (2), 139-145 (2001).
- Meaney, M., Haughey, S., Brennan, G. P., Fairweather, I. A scanning electron microscope study on the route of entry of clorsulon into the liver fluke, Fasciola hepatica. Parasitol. Res. 95 (2), 117-128 (2005).
- Sundarasekar, J., Sahgal, G., Subramaniam, S. Anti-candida activity by Hymenocallis littoralis extracts for opportunistic oral and genital infection Candida albicans. Bangladesh J. Pharmacol. 7 (3), 211-216 (2012).
- Benhamou, N., Rey, P., Picard, K., Tirilly, Y. Ultrastructural and cytochemical aspects of the interaction between the mycoparasite Pythium oligandrum and soilborne plant pathogens. Phytopathology. 89 (6), 506-517 (1999).
- Singh, A., et al. First evidence of putrescine involvement in mitigating the floral malformation in mangoes: A scanning electron microscope study. Protoplasma. 251 (5), 1255-1261 (2014).
- Xiang, C., et al. Fine mapping of a palea defective 1 (pd1), a locus associated with palea and stamen development in rice. Plant Cell Rep. 34 (12), 2151-2159 (2015).
- Mendoza, L., Hernandez, F., Ajello, L. Life cycle of the human and animal oomycete pathogen Pythium insidiosum. J. Clin. Microbiol. 31 (11), 2967-2973 (1993).
- Bello, M. A., Rudall, P. J., González, F., Fernández, J. L. Floral morphology and development in Aragoa (Plantaginaceae) andrelated members of the order Lamiales. Int. J. Plant Sci. 165 (5), 723-738 (2004).
- Bello, M. A., Hawkins, J. A., Rudall, P. J. Floral morphology and development in Quillajaceae and Surianaceae (Fabales), the species-poor relatives of Leguminosae and Polygalaceae. Ann. Bot. 100 (4), 1491-1505 (2007).
- Bello, M. A., Hawkins, J. A., Rudall, P. J. Floral ontogeny in Polygalaceae and its bearing on the homologies of keeled flowers in Fabales. Int. J. Plant Sci. 171 (5), 482-498 (2010).
- Bello, M. A., Alvarez, I., Torices, R., Fuertes-Aguilar, J. Floral development and evolution of capitulum structure in Anacyclus (Anthemideae, Asteraceae). Ann. Bot. 112 (8), 1597-1612 (2013).
- Bello, M. A., Martínez-Asperilla, A., Fuertes-Aguilar, J. Floral development of Lavatera trimestris and Malva hispanica reveals the nature of the epicalyx in the Malva generic alliance. Bot. J. Linn. Soc. 181 (1), 84-98 (2016).
- Calonge, F. D., Martínez, A. J., Falcó, I., Samper, L. E. Phellorinia herculanea f. stellata f. nova encontrada en España. Bol. Soc. Micol.Madrid. 35 (1), 65-70 (2011).
- Liu, Y., et al. Deciphering microbial landscapes of fish eggs to mitigate emerging diseases. ISME J. 8 (10), 2002-2014 (2014).
- Sandoval-Sierra, J. V., Diéguez-Uribeondo, J. A comprehensive protocol for improving the description of Saprolegniales (Oomycota): two practical examples (Saprolegnia aenigmatica sp. nov. and Saprolegnia racemosa sp. nov.). PLOS one. , (2015).
- Endress, P. K. Zur vergleichenden Entwicklungsmorphologie, Embryologie und Systematik bei Laurales. Bot. Jahrb. Syst. 92 (2), 331-428 (1972).
- Tucker, S. Floral development in Saururus cernuus (Saururaceae):1. Floral initiation and stamen development. Am. J. Bot. 62 (3), 993-1005 (1975).
- Endress, P. K., Matthews, M. L. Progress and problems in the assessment of flower morphology in higher-level systematics. Plant Syst. Evol. 298 (2), 257-276 (2012).
- Beakes, G. W., Glockling, S. L., Sekimoto, S. The evolutionary phylogeny of the oomycete "fungi". Protoplasma. 249 (1), 3-19 (2012).
- Romansic, J. M., et al. Effects of the pathogenic water mold Saprolegnia ferax on survival of amphibian larvae. Dis. Aquat. Organ. 83 (3), 187-193 (2009).
- van West, P. Saprolegnia parasitica, an oomycete pathogen with a fishy appetite: new challengues for an old problem. Mycologist. 20 (3), 99-104 (2006).
- Johansen, D. A. . Plant microtechnique. , (1940).
- Unestam, T. Studies on the crayfish plague fungus Aphanomyces astaci. Some factors affecting growth in vitro. Physiol. Plantarum. 18 (2), 483-505 (1965).
- Cerenius, L., Söderhäll, K. Repeated zoospore emergence from isolated spore cysts of Aphanomyces astaci. Exp. Mycol. 8 (4), 370-377 (1984).
- Diéguez-Uribeondo, J., Cerenius, L., Söderhäll, K. Repeated zoospore emergence in Saprolegnia parasitica. Mycol. Res. 98 (7), 810-815 (1994).
- Söderhäll, K., Svensson, E., Unestam, T. Chitinase and protease activities in germinating zoospore cysts of a parasitic fungus, Aphanomyces astaci, Oomycetes. Mycopathologia. 64 (1), 9-11 (1978).
- Echlin, P. . Handbook of sample preparation for scanning electron microscopy and X-Ray Microanalysis. , (2009).
- Osumi, M., et al. Preparation for observation of fine structure of biological specimens by high-resolution SEM. Microscopy. 32 (4), 321-330 (1983).
- Rezinciuc, S. . The Saprolegniales morpho-molecular puzzle: an insight into markers identifying specific and subspecific levels in main parasites. , (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved