需要订阅 JoVE 才能查看此. 登录或开始免费试用。
Method Article
人类诺沃克病毒的表面上检测拭子采样方法
摘要
A macrofoam based sampling methodology was developed and evaluated for the detection and quantification of norovirus on environmental hard surfaces.
摘要
诺如病毒的人是流行性和世界各地的零星胃肠炎的主要原因。因为大多数感染或者直接经由人对人的路线通过环境表面或食物传播或间接地受污染的非生物媒介和无生命的表面是用于病毒的期间诺罗病毒爆发的传播重要的车辆。
我们开发和评估使用大泡拭子用于检测和从硬表面人类诺如病毒的打字的协议。纤维拭子或防静电抹布相比,大泡拭子允许从高达700 cm 2的马桶座面恢复病毒(范围1.2-33.6%)。该协议包括用于病毒从拭子提取和使用旋转柱的病毒RNA的进一步浓缩的步骤。总共127已经从在游船和长期护理设施的表面,其中诺罗病毒性胃肠炎已经收集到217拭子样品(58.5%)报道药检呈阳性被RT-qPCR的诺如病毒GII。这些29(22.8%)的可以成功基因分型。总之,检测使用我们开发可以帮助确定环境污染的暴发期间水平以及检测病毒的当临床样品是不可用的协议环境表面上诺罗病毒的;它也可能有利于整治策略的有效性进行监测。
引言
诺如病毒的人是流行性和散发性急性胃肠炎全世界1,2,3的主要原因。该病毒是极具传染性并通过直接的人发生互动的人或间接通过受污染的食物,水或环境表面接触传播。诺如病毒可棚长时间和长期的环境表面上的病毒的生存已记载1,2,3。在高峰脱落,十亿病毒颗粒被每克释放粪便和呕吐物还含有足够数量的病毒颗粒中,以引起感染4,5,6,7,8,EF"> 9,10,另外,可以很容易地发生无生命的表面和人的皮肤之间的病毒传输2,11,12,因此,环境污染的监测可有助于爆发调查和评估的清理的有效性和消毒程序。
几个环境取样协议已经用于检测轮状病毒了描述,大肠杆菌噬菌体MS2,猫杯状病毒(FCV),和噬菌体P22 13,14,15,16。然而,在这些研究中,包括快速干燥(<1小时)和小的表面区域中所述的验证条件(25×100厘米2)时,可能不能充分代表字段设置。此外,预期ENVIRO的低的污染水平nmental表面需要是能够检测很少的病毒颗粒的协议。
我们开发了诺如病毒的检测和分型基于大泡,表面取样方法。这种方法已经在几次爆发诺沃克病毒被证实。该协议包括1)如何收集环境表面拭子样本(2)如何收集和运输到实验室过程中最好保持样本的完整性,以及3)诺如病毒的实验室测试和打字。
研究方案
1.棉签取样的场
- 穿一双干净的手套。
- 测量取样区域的大小,而无需使用卷尺或直尺接触到表面。尝试尽可能准确地估计该地区并填写报告表(补充表1)。
- 检查可能的泄漏和标签样本运输袋,包棉签拭子套件。
- 移动拭子穿过取样面积如下:在水平方向的行程,在垂直方向的一个行程,并在对角方向的一个笔划。不要擦拭表面面积超过700 平方厘米大。
- 将每个拭子插入管并拧紧瓶盖。
2.储存和运输拭子的实验室
- 保持在4℃拭子长达48小时。如果需要较长时间的存储,存储所述拭子在-20℃(或-70℃)。
- 保持管在0-4°C( 即。在隔热容器运送至实验室和船舶中收集的24-48小时内使用冷包)。
3.病毒浓度,病毒RNA提取纯化
注意:所有的离心步骤使用台式离心机以5,000×g离心5分钟,在室温下,除非另有说明。与通用核酸提取(UNEX)缓冲区时要格外小心。戴上护目镜或面罩。
- 标签1个15毫升管,并为每个样品一种RNA的中型柱。包括在每个实验中的负提取控制。
- 要为10拭子准备裂解液,将25ml UNEX缓冲与25毫升的PBST混合(PBS pH值7.2含0.02%吐温80)。
- 添加噬菌体MS2悬浮液(10 6 PFU /微升)50微升到50毫升的裂解溶液。
- 将棉签在15毫升管加入5 ml裂解液。混合并孵育在室温下10分钟。
- 5毫升100%乙醇添加至每个管并涡旋10秒。
- 小心取出15毫升管拭子(它包含UNEX缓冲)轻轻压在管的一侧以除去多余的液体,然后丢弃棉签。其余各卷应该是9-10毫升之间。
4.中型柱病毒核酸提取
- 小心地从步3.6 4.5毫升UNEX /乙醇混合物转移到一个中型柱,离心,弃滤液。
- 装载从同一混合物另4.5毫升到同一列中,离心,并弃去滤液。
- 对于第一次洗涤,添加3.5毫升70%乙醇到中型柱,离心并弃去滤液。
- 对于第二次洗涤,加入另一3.5毫升70%乙醇到中型柱。离心并弃去滤液。
- 对于干式旋,离心迷笛列,除去乙醇所有痕迹(可产生负面影响您的病毒RNA恢复)。
- 放置南部列在一个新的15ml离心管中。加入250微升洗脱缓冲到中型柱;离心分离得到最高的病毒RNA恢复之前等待1分钟。
- 存储所提取的核酸在-70℃或立即进行到步骤5。
5.浓缩病毒核酸RNA使用清洁和集中套件
- 添加的RNA结合缓冲液在上述步骤4.7和涡洗脱10秒RNA的250μL500μL。
- 加入750μL100%乙醇,涡旋10秒。
- 标记每个样品离心柱。加载750微升样品到离心柱。
- 离心旋转柱以12,000 xg离心1分钟。丢弃的流量通过。
- 加载剩余的750微升到旋转柱和离心机以12,000 xg离心1分钟。
- 为预洗,以12,000 xg离心400μL的RNA的准备缓冲器添加到每个旋转柱和离心1分钟。丢弃的流量通过。
- 对于第一次洗涤,以12,000 xg离心添加800μL的RNA洗涤缓冲液到每个旋转柱和离心1分钟。丢弃的流量通过。
- 对于第二次洗涤,加入400μL的RNA的洗涤缓冲液以12,000 xg离心旋转柱和离心1分钟。丢弃的流量通过。
- 对于干式纺丝,离心旋转柱以12,000 xg离心以除去洗涤缓冲液的所有痕迹2分钟。
- 仔细离心柱转移到一个干净的1.5 ml离心管。
- 添加25微升洗脱缓冲液到旋转柱并孵育在室温下1分钟。这会增加从塔病毒RNA的回收。
- 通过以10,000 xg离心离心旋转柱1分钟收集RNA。
- 无论是在-70℃直接进行RT-qPCR的(步骤6)或商店的RNA。
6.基因型组I的Mutiplex RT-qPCR的检测,和II诺如病毒,和大肠杆菌噬菌体的MS 2(补充表2)
- 清洁的工作表面,pipettES,并与核糖核酸酶净化解决方案的离心机,以减少可能的污染。
- 在冰上解冻RT-qPCR的试剂。在冰上解冻RNA(在一个单独的区域/间)。
- 确定反应的数量和它们添加更多的至少10%( 例如 ,如果需要10反应,使11个主混合物)。
- 涡单个主结构组件,除了25X RT-qPCR的酶,5秒。小心通过用手指轻弹管混合酶。
- 短暂离心母液成分为5秒。
- 根据试剂盒说明书准备诺如病毒GI和GII的实时荧光定量RT-PCR检测预混和添加特定的诺如病毒的寡核苷酸引物和探针在1.5 ml离心管(补充表3)。
- 吹打5-10倍上下混合母液(不推荐涡旋)。等分试样22微升在实时PCR的96孔板的每个孔中的主混合物。
- 涡样品RNA5秒,并进行了简短(5秒)离心收集。
- 加入样品RNA和GI和GII阳性对照3μL到RT-qPCR的板(遵循从表2模板)。添加核酸酶Freewater的3微升在无模板对照(NTC)的孔中。
- 密封用光学粘合剂膜实时板。
- 在1300 xg离心仔细离心实时板1分钟,以除去任何气泡或液滴可能存在于孔中。
- 设置了实时PCR仪和设置下列热循环条件:1)10分钟的RT步骤。在45℃(2)激活Taq聚合酶,10分钟。在95℃,(3)在95℃下45个循环的15秒,60秒,在60℃。
7.诺如病毒的定量的拭子样品
- 检查的阳性和阴性对照的结果来验证,RT-qPCR的结果( 补充表3)。
- 确定每个标准曲线是否满足可接受值在补充表2中给出,并计算使用公式1的标准曲线的总体标准偏差。
(公式1)%的效率= 100×10(平均Ct值截距)/斜率 。 - 如果在PCR仪软件不计算斜率为每个标准曲线,确定斜率和R使用统计软件2值回归( 例如 Excel,SPSS,SAS或)。此外,计算公式如下I标准曲线%的效率
- 记录由热循环仪软件基于满足补充表4规定的标准,标准曲线所有测试样本计算出来的RNA拷贝数。重新运行与标准曲线牛逼帽子的样品不符合标准或有假阳性对照。检查大肠杆菌噬菌体MS2,其中加入作为内部对照来监测PCR抑制的Ct值。
- 确定RNA拷贝p的总数由总RNA洗脱剂的体积(25至50μl)的该用于RT-qPCR的反应中的RNA(3-5微升)的比例,在步骤7.2计算出的RNA拷贝数乘法器样品。可替代地,通过用分析的对象的表面积(厘米2)除以总RNA拷贝数计算出的RNA浓度。
8.基因分型实时定量RT-PCR阳性样品由赫米嵌套常规PCR扩增
- 制备第一轮主混合物的每个引物组的RT-PCR测定的( 补充表1,2和5)。
- 诺如病毒添加RNA阳性的5微升至预混20μL。
- 在下列条件下运行的RT-PCR:在42℃(2)激活Taq聚合酶,15分钟30分钟(1)RT步骤在95℃,和(3)在95℃下的30秒40个循环,30秒,在50℃,并在72℃下60秒。后40个循环,孵育在72℃另外10分钟。
- 准备为每个引物组的RT-PCR测定的( 补充表2和5)在第二轮脑膜混合物。
- 加入23μl反应混合液,并添加第一轮RT-PCR产物(从第一轮)2微升,每管(最好是第一轮产品应在无RNase水稀释1/10)。
- 重复步骤8.3。
- 制备在100ml 1×Tris-乙酸EDTA的2%琼脂糖凝胶(40毫摩尔Tris,20mM的乙酸,和1mM EDTA,pH为8.3)。通过在微波炉中加热该混合物琼脂糖溶解后,将混合物冷却到60-70℃后加入10微升每100mL制得的凝胶的核酸染色,倒入该凝胶并插入梳子。让凝胶沉降至少30分钟。
- 混合15微升各RT-PCR产物与6×上样染料的3微升。负荷各样品的15微升和electrophorese在100V琼脂糖凝胶1小时。
- 从凝胶合适的大小(330个基点GI和341个基点GII)的消费目标RT-PCR片段d使用商业凝胶提取试剂盒纯化RNA。纯化的PCR产物现在可用于Sanger测序。
结果
图1呈现拭子采样协议的流程图。这个协议包括四个主要步骤; 1)样品的采集,2)样品存储和运输,3)病毒RNA纯化和浓缩和4),RT-qPCR分析和基因分型。
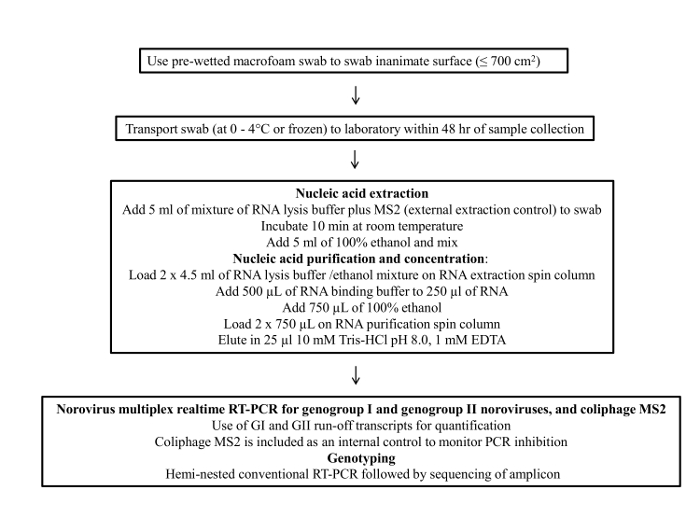
图1:流量为诺如病毒的环境表面取样最终协议的图表
讨论
诺如病毒有18和10 3病毒颗粒20之间的50%的人感染剂量。因此,即使表面的低水平的污染可能对公共健康危险。拭子采样协议的若干方面进行了评价,包括:1)不同拭子的材料,2)在运输过程中贮存条件拭子,3)病毒RNA的浓度,和4)噬菌体MS2作为内提取的控制。
直到最近,才由棉,涤纶,锦纶和抗静电擦拭)制成拭子的性能进行了评估,并提出适合...
披露声明
Authors have no conflicting interest. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
致谢
The authors have no acknowledgements.
材料
| Name | Company | Catalog Number | Comments |
| Generic name for kits | |||
| Macrofoam swab | Premoistened EnviroMax Swab kit | Puritan | 2588060PFUW |
| RNA Lysis buffer | CDC UNEX buffer | Microbiologics | Cat No MR0501 |
| RNA extraction spin column | Midi column | Omega Biotek | Cat No R6664-02 |
| RNA purification spin column | Zymol RNA Clean and Concentrator kit | Zymo Research | Cat No R1016 |
| Real time RT-PCR kit | AgPath kit One-Step RT-PCR Kit | Life Technologies | Cat No 4387391 |
| Conventional RT-PCR kit | Qiagen one step RT-PCR kit | Qiagen kit | Cat No 210212 |
| Gel extraction kit | Qiagen QIAquick gel extraction kit | Qiagen kit | Cat No 28704 or 28706 |
| Coliphage MS2 | ATCC | Cat No 15597-B1 | |
| RNA run-off transcripts | |||
| Realtime PCR platform | Applied Biosystems | Model ABI 7500 | |
| Optical 96-well reaction plate | Thermo Scientific | Cat No 4316813 | |
| MicroAmp Clear Adhesive Film | Thermo Scientific | Cat No 4306311 |
参考文献
- Isakbaeva, E. T., et al. Norovirus transmission on cruise ship. Emerg. Infect. Dis. 11, 154-158 (2005).
- Lopman, B. A., Gastañaduy, P., Park, G. W., Hall, A. J., Parashar, U. D., Vinjé, P. Environmental transmission of norovirus gastroenteritis. Curr. Opin. Virol. 2 (1), 1-7 (2011).
- Malek, M., et al. Outbreak of norovirus infection among river rafters associated with packaged delicatessen meat, Grand Canyon, 2005. Clin Infect Dis. 48 (1), 31-37 (2009).
- Atmar, R. L., et al. Norwalk virus shedding after experimental human infection. Emerg. Infect. Dis. 14 (10), 1553-1557 (2008).
- Glass, R. I., Parashar, U. D., Estes, M. K. Norovirus gastroenteritis. N. Engl. J. Med. 361 (18), 1776-1785 (2009).
- Park, G. W., et al. Evaluation of a New Environmental Sampling Protocol for Detection of Human Norovirus on Inanimate Surfaces. Appl. Environ. Microbiol. 81 (17), 5987-5992 (2015).
- Barker, J., Jones, M. V. The potential spread of infection caused by aerosol contamination of surfaces after flushing a domestic toilet. J. Appl. Microbiol. 99, 339-347 (2005).
- Tung-Thompson, G., Libera, D. A., Koch, K. L., de Los Reyes, F. L., Jaykus, L. A. Aerosolization of a Human Norovirus Surrogate, Bacteriophage MS2, during Simulated Vomiting. PloS one. 10, 0134277 (2015).
- Atmar, R. L., et al. Determination of the 50% human infectious dose for Norwalk virus. J. Infect. Dis. 209 (7), 1016-1022 (2014).
- Petrignani, M., van Beek, J., Borsboom, G., Richardus, J. H., Koopmans, M. Norovirus introduction routes into nursing homes and risk factors for spread: a systematic review and meta-analysis of observational studies. J. Hosp. Infect. 89 (3), 163-178 (2015).
- . Centers for Disease Control Prevention. Norovirus outbreak in an elementary school--District of Columbia, February 2007. MMWR. Morb. Mortal. Wkly. Rep. 56 (51-52), 1340-1343 (2008).
- Cheesbrough, J. S., Barkess-Jones, L., Brown, D. W. Possible prolonged environmental survival of small round structured viruses. J. Hosp. Infect. 35, 325-326 (1997).
- Julian, T. R., Tamayo, F. J., Leckie, J. O., Boehm, A. B. Comparison of surface sampling methods for virus recovery from fomites. Appl. Environ. Microbiol. 77, 6918-6925 (2011).
- Taku, A., et al. Concentration and detection of caliciviruses from food contact surfaces. J. Food. Prot. 65, 999-1004 (2002).
- Scherer, K., Ellerbroek, L., Schulenburg, J., Johne, R., Klein, G. Application of a swab sampling method for the detection of norovirus and rotavirus on artifically contaminated food and environmental surfaces. Food. Environ. Virol. 1 (42), 42-49 (2009).
- Herzog, A. B., et al. Evaluation of sample recovery efficiency for bacteriophage P22 on fomites. Appl. Environ. Microbiol. 78, 7915-7922 (2012).
- Vega, E., et al. CaliciNet: A Novel Surveillance Network for Norovirus Gastroenteritis Outbreaks in the United States. Emerging Infectious Diseases. 17 (8), 1389-1395 (2011).
- Rolfe, K. J., et al. An internally controlled, one-step, real-time RT-PCR assay for norovirus detection and genogrouping. J Clin Virol. 39 (4), 318-321 (2007).
- Kittigul, L., et al. Norovirus GII-4 2006b variant circulating in patients with acute Thailand during a 2006-2007 study. J. Med. Virol. 82 (5), 854-860 (2010).
- Teunis, P. F., et al. Norwalk virus: how infectious is it. J. Med. Virol. 80 (8), 1468-1476 (2008).
- Wollants, E., et al. Evaluation of a norovirus sampling method using sodium dodecyl sulfate/EDTA-pretreated chromatography paper strips. J. Virol. Methods. 122, 45-48 (2004).
- Weir, M. H., Shibata, T., Masago, Y., Cologgi, D., Rose, J. B. The Effect of Surface Sampling and Recovery of Viruses and Non-Spore Forming Bacteria on a QMRA Model for Fomites. Environ. Sci. Technol. 50 (11), 5945-5952 (2016).
- . Microbiology of food and animal feed-Horizontal method for determination of hepatitis A virus and norovirus in food using real-time RT-PCR. International Organization for Standardization (ISO). , (2013).
- Huslage, K., Rutala, W. A., Sickbert-Bennett, E., Weber, D. J. A quantitative approach to defining "high-touch" surfaces in hospitals. Infect. Control. Hosp. Epidemiol. 31 (8), 850-853 (2010).
- Wu, H. M., et al. A norovirus outbreak at a long-term-care facility: the role of environmental surface contamination. Infect. Control. Hosp. Epidemiol. 26 (10), 802-810 (2005).
- Ikner, L. A., Gerba, C. P., Bright, K. R. Concentration and recovery of viruses from water: a comprehensive review. Food Environ. Virol. 4 (2), 41-67 (2012).
- Gallimore, C. I., et al. Environmental monitoring for gastroenteric viruses in a pediatric primary immunodeficiency unit. J. Clin. Microbiol. 44 (2), 395-399 (2006).
- Ganime, A. C., et al. Dissemination of human adenoviruses and rotavirus species A on fomites of hospital pediatric units. Am J Infect Control. , (2016).
- Verani, M., Bigazzi, R., Carducci, A. Viral contamination of aerosol and surfaces through toilet use in health care and other settings. Am J Infect Control. 42 (7), 758-762 (2014).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可探索更多文章
This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。