A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Synthesis and Testing of Supported Pt-Cu Solid Solution Nanoparticle Catalysts for Propane Dehydrogenation
In This Article
Summary
A convenient method for the synthesis of 2 nm supported bimetallic nanoparticle Pt-Cu catalysts for propane dehydrogenation is reported here. In situ synchrotron X-ray techniques allow for the determination of the catalyst structure, which is typically unobtainable using laboratory instruments.
Abstract
A convenient method for the synthesis of bimetallic Pt-Cu catalysts and performance tests for propane dehydrogenation and characterization are demonstrated here. The catalyst forms a substitutional solid solution structure, with a small and uniform particle size around 2 nm. This is realized by careful control over the impregnation, calcination, and reduction steps during catalyst preparation and is identified by advanced in situ synchrotron techniques. The catalyst propane dehydrogenation performance continuously improves with increasing Cu:Pt atomic ratio.
Introduction
Propane dehydrogenation (PDH) is a key processing step in the production of propylene, taking advantage of shale gas, the fastest growing source of gas in the country1. This reaction breaks two C-H bonds in a propane molecule to form one propylene and molecular hydrogen. Noble metal catalysts, including Pd nanoparticles, exhibit poor selectivity for PDH, breaking the C-C bond to produce methane with a high yield, with the concomitant production of coke, leading to catalyst deactivation. Recent reports showed that selective PDH catalysts could be obtained by the addition of promoters like Zn or In to Pd2,3,4. The promoted catalysts are near 100% selective to PDH, as opposed to less than 50% for monometallic Pd nanoparticles of the same size. The great improvement in selectivity was attributed to the formation of PdZn or PdIn intermetallic compound (IMC) structures on the catalyst surface. The ordered array of two different types of atoms in the IMCs geometrically isolated the Pd active sites with non-catalytic Zn or In atoms, which turned off the side reactions catalyzed by an ensemble (group) of neighboring Pd active sites.
Platinum has the highest intrinsic selectivity among noble metals for propane dehydrogenation, but it is still not satisfactory for commercial use1. Typically, Sn, Zn, In, or Ga is added as promoter for Pt5,6,7,8,9,10,11,12,13. Based on the idea that geometric active site isolation contributes to high selectivity, any non-catalytic element forming an alloy structure with Pt, such as Cu, should also potentially promote catalyst performance14. Several previous studies suggested that the addition of Cu indeed improved the PDH selectivity of Pt catalysts15,16,17,18. Nevertheless, no direct evidence has been reported to determine whether Pt and Cu form bimetallic nanoparticles or ordered structures, which is crucial to understanding the promotional effect of Cu. In the binary phase diagram of Pt-Cu, two different structure types are possible over a wide composition range16,18: intermetallic compound, in which Pt and Cu each occupy specific crystal sites, and solid solution, in which Cu randomly substitutes in the Pt lattice. IMCs form at low temperature and transform into solid solution at around 600 - 800 °C for bulk materials14. This transformation temperature may be lower for nanoparticles, near the reaction temperature of PDH (i.e. 550 °C). Therefore, it is essential to investigate the atomic order of Pt-Cu under reaction conditions. For supported nanoparticles with small particle sizes, it is very challenging to obtain meaningful structural information using laboratory instruments19. The limited repetition of unit cells leads to very broad diffraction peaks with very low intensities. Because of the high fraction of surface atoms in nanoparticles 1 - 3 nm in size, which are oxidized in air, diffraction must be collected in situ using high-flux X-ray, typically available with synchrotron techniques.
The previously reported Pt-Cu PDH catalysts were all larger than 5 nm in size15,16,17,18. However, for noble metal nanoparticle catalysts, there is always a strong desire to maximize catalytic activity per unit cost by synthesizing catalysts with high dispersions (typically around or less than 2 nm in size)19. Though the preparation of bimetallic nanoparticles of this size is possible by standard impregnation methods, rational control over the procedures is necessary. The metal precursors, pH of the impregnating solution, and support type need to be controlled to optimize the anchoring of the metal species onto high-surface area supports. The subsequent calcination and reduction heat treatments should also be carefully controlled to suppress the growth of the metallic nanoparticles.
This article covers the protocol for the synthesis of supported 2 nm Pt-Cu bimetallic nanoparticle catalysts and for the testing of their propane dehydrogenation performance. The structure of the catalysts is investigated by Scanning Transmission Electron Microscopy (STEM), in situ synchrotron X-ray Absorption Spectroscopy (XAS), and in situ synchrotron X-ray diffraction (XRD), which help elucidate the improved catalyst performance upon the introduction of Cu.
Protocol
1. Synthesis of Supported 2 nm Pt-Cu Bimetallic Nanoparticle Catalysts
- Preparation of metal precursor solution
- Dissolve 0.125 g of copper nitrate trihydrate (Cu(NO3)2·3H2O) in 1 mL of water to achieve a sky blue solution.
Caution: Use protective gloves when handling chemicals. - Add ammonia dropwise to the copper nitrate solution, forming dark blue precipitates of copper hydroxide.
Caution: Use a fume hood for handling bases and volatile chemicals. - Keep adding ammonia until the dark blue precipitates dissolve to form a dark blue solution and the pH >10.
- Add 0.198 g of tetraammineplatinum nitrate ((NH3)4Pt(NO3)2) to the solution and additional water so that the total volume of the solution is 3.5 mL. Add additional ammonia if necessary to keep the pH of the solution greater than 10.
- Heat the solution to 70 °C until all the tetraammineplatinum nitrate salts are dissolved in the solution. Allow the solution cool to RT.
- Dissolve 0.125 g of copper nitrate trihydrate (Cu(NO3)2·3H2O) in 1 mL of water to achieve a sky blue solution.
- Co-impregnation of metal precursor solution
- Prior to catalyst preparation, determine the impregnating pore volume of the silica support. Carefully weigh approximately 5 g of dry silica into a weighting dish. While mixing, add H2O dropwise until the silica is completely wet, but with no excess solution. Reweigh the wet silica. Divide the grams of added water by the grams of sample to calculate the pore volume.
- Add the dissolved metal precursor solution a few drops at a time to 5 g of high-porous silica (SiO2) in a ceramic evaporating dish and stir gently to break up the particles that stick together to achieve a homogeneous distribution of the solution.
NOTE: The white silica will turn dark blue once it adsorbs all 3.5 mL of metal precursor solution.- Make sure that the texture of the silica particles remains like that of dry sand. Avoid the accumulation of excess solution during impregnation.
- Place the impregnated Pt-0.7Cu/SiO2 catalyst precursor into a drying oven and dry it at 125 °C O/N.
- Calcination and reduction
- Calcine the catalyst in a furnace at 250 °C with a 5 °C/min ramp rate in air for 3 h.
NOTE: Calcination at higher temperatures generally leads to larger Pt nanoparticles. - Place a 2 cm layer of quartz wool in the middle of a 1" quartz tube reactor and load the calcined Pt-0.7Cu/SiO2 catalyst into the tube through a plastic funnel. Place the tube in a clamshell temperature-programmed furnace.
- After purging the tube with N2 for 5 min at RT, start to flow H2 (at RT) at the same flow rate as N2 (100 ccm) to reduce the Pt-0.7Cu/SiO2 catalyst.
- Increase the temperature to 150 °C with a 5 °C/min ramp rate and hold for 5 min.
- Start slow ramping the temperature at a rate of 2.5 °C/min to 250 °C. Hold the temperature for 15 min at every 25 °C.
NOTE: Other metals may require lower or higher temperatures of reduction. The exact temperature can generally be determined by examining color changes of the catalyst (e.g., from blue to black) for Pt-Cu. - Ramp to 550 °C (or the reaction temperature, if higher) at 10 °C/min and stay for 30 min to complete the reduction. Purge with N2 and cool to room temperature.
- Unload the Pt-0.7Cu/SiO2 catalysts and store in a vial for future use.
NOTE: Repeat similar synthesis procedures using different amount of Cu(NO3)2·3H2O and (NH3)4Pt(NO3)2 to prepare the other Pt-X Cu/SiO2 catalysts (X = 0.7, 2.3, and 7.3 and stands for Cu:Pt atomic ratios) and Pt/SiO2 catalysts.
- Calcine the catalyst in a furnace at 250 °C with a 5 °C/min ramp rate in air for 3 h.
2. Propane-dehydrogenation Performance Test
- Catalyst loading
- Take a 3/8" quartz tube reactor and place a 1 cm layer of quartz wool against the dimple in the middle.
Caution: Use protective gloves when handling quartz wool, since the fine needles can get imbedded in the skin. - Weigh 40 mg of Pt-0.7Cu/SiO2 catalyst and 960 mg of the silica for dilution. Mix the particles (1 g total weight) in an empty vial.
- Use a plastic funnel to load all the catalyst mixture into the reaction tube. Wipe the outer wall of both tube ends with lint-free wipes to remove any dirt to get a good seal with the O-ring.
- Put the tube fittings onto both ends of the quartz reactor tube and attach them to the reactor system equipped with a clamshell furnace.
- Take a 3/8" quartz tube reactor and place a 1 cm layer of quartz wool against the dimple in the middle.
- Leak test and catalyst pretreatment
- Turn on 50 cm3/min N2 flow through the tube reactor. After 1 min, close the ball valve on the reactor outlet. Wait for the system pressure to increase to 5 psig. Close the ball valve on the inlet N2 line to stop N2 flow and seal the reactor system.
- Wait for 1 min and record the pressure read from the gauge. If the pressure drops, open the ball valve on the reactor outlet to release the pressure and re-attach the fittings. If not, first open the ball valve on the reactor outlet to release the pressure before restarting the N2 flow by turning on the ball valve on the inlet N2 line to purge the system for 1 min.
- Start flowing 50 cm3/min of 5% H2/N2 for catalyst reduction before running a reaction and stop the N2 flow. Start heating the tube to the reaction temperature of 550 °C, with a rate of 10 °C/min. Wait for 30 min after the furnace reaches the set point and allow the system temperature to stabilize at the target temperature.
- Propane dehydrogenation reaction testing
- Start the gas chromatograph (GC) in the reactor system and select the proper method for gas component analysis.
- Switch the reactor gas flow to a bypass line. Flow 100 cm3/min of 5% propane/N2 and 100 cm3/min of 5% H2/N2. Wait for 1 min so that the propane flow stabilizes and inject the bypass flow into GC as a reference sample.
- Switch the gas flow back to the reactor tube line to start the reaction and record the time.
- After the reaction runs for 4 min, inject the reactor outlet gas flow (a GC sample) into the GC to get the outlet gas component information. Inject samples every 4 min and run the test until the conversion reaches steady state or the conversion is very low.
- Use the corresponding peak analysis software to analyze each peak.
- Click to select the start and end points of the peak. Use the integrate function to get the peak area. Write down the peak area for the propane (C3H8) reactant; the propylene (C3H6) product; and the side products, methane (CH4), ethane (C2H4), and ethylene (C2H6).
NOTE: For each injection, a GC pattern with multiple peaks is obtained whose area relates to the number of moles of different gas species.
- Click to select the start and end points of the peak. Use the integrate function to get the peak area. Write down the peak area for the propane (C3H8) reactant; the propylene (C3H6) product; and the side products, methane (CH4), ethane (C2H4), and ethylene (C2H6).
- Convert the peak area to the number of moles for each species using the response factor. Determine the propane conversion and propylene selectivity at the time for each sample according to the following formulas:
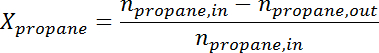
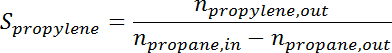
where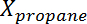 is the conversion of propane,
is the conversion of propane, 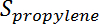 is the propylene selectivity,
is the propylene selectivity, 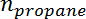 is the number of moles of propane, and
is the number of moles of propane, and 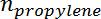 is the number of moles of propylene.
is the number of moles of propylene. - Obtain the initial conversion and selectivity value at t = 0 by extrapolating the measured conversion and selectivity versus time on stream using an exponential fit.
- Start the gas chromatograph (GC) in the reactor system and select the proper method for gas component analysis.
- Post-reaction
- Stop heating the reactor by turning off the temperature program. Switch the gas flow to 10 cm3/min N2.
- Switch the gas chromatograph back to standby method to reduce the flow rate of the carrier gas.
- Unload the used catalyst from the quartz fix-bed reactor after cooling to room temperature. Collect the catalyst weight in a designated waste disposal area.
3. Characterization of Catalyst Samples
- Scanning Transmission Electron Microscopy4,20
- Load the catalyst in a mortar and grind it into less than 100 mesh powder using a pestle.
- Disperse about 30 mg of catalyst powder into about 5 mL of isopropyl alcohol in a small vial. Shake the vial for full mixing and then let the vial sit for 5 min to allow for the deposition of the relatively large particles.
NOTE: The obtained suspension should contain very small particles of supported catalysts. - Place a Au TEM ready grid on an evaporating dish. Heat the dish to 80 °C on a hot plate. Add three drops of the catalyst suspension to the grid.
NOTE: The isopropyl alcohol will evaporate quickly and leave the catalyst particles on the grid. - Load the grid onto the sample holder for electron microscopy sample imaging.
- In situ X-ray absorption spectroscopy3,4,19,20
- Load the catalyst into a mortar and grind it into less than 100 mesh powder using a pestle. Load the fine powders into a die set and press it with the fingers to form a self-supporting wafer.
- Load a ~100 mg sample into the sample holder.
- Place the sample holder into a quartz tube reactor and pretreat the sample by reducing it in 50 cm3/min 3% H2/He.
- After cooling to RT, seal the tube and transfer it to the synchrotron beamline to collect the XAS data.
- In situ X-ray diffraction 19,20
- Load the catalyst in a mortar and grind it into less than 100 mesh powder using a pestle.
- Press a thin wafer using a standard 7 mm diameter die set.
NOTE: The die set contains a female piece and top and bottom male pieces.- Attach the bottom male piece to the female part. Load the sample onto the polished surface of the bottom part. Attach the top male piece and transfer the die set to the sample stage of the press. Press with appropriate strength.
- Unload the wafer and transfer it to the ceramic cup of the specialized sample stage (see the Table of Materials). Seal the stage and fix it on the sample table on the beamline.
- Reduce the sample by flowing and ramping the temperature to 550 °C. Collect the in situ X-ray diffraction data under 3% H2/He gas flow at 550 °C and after cooling down to RT20.
Results
The propylene selectivity versus time for Pt and Pt-Cu catalysts measured at an initial propane conversion of about 20% is presented in Figure 1A. Pt catalyst has an initial selectivity of 61%, which increases to about 82% with time on-stream as the catalyst deactivates for 1h. The Pt-0.7Cu catalyst shows a better initial propylene selectivity of 72%. For Pt-2.3Cu and Pt-7.3Cu catalysts, their initial selectivity reach 90% and 96%, respectively, and are maint...
Discussion
The Pt-Cu catalysts prepared in this work contain uniform nanoparticles around 2 nm in size, similar to heterogeneous catalysts qualified for industrial application. All the Pt and Cu precursors form bimetallic structures, as opposed to separate monometallic particles. This bimetallic interaction and small particle size are realized by careful control over the synthesis procedures. The impregnation process makes use of the Strong Electrostatic Adsorption (SEA) between metal ions and the surface of certain oxide supports<...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the School of Chemical Engineering, Purdue University. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Basic Energy Sciences, under contract no. DE-AC02-06CH11357. MRCAT operations, beamline 10-BM are supported by the Department of Energy and the MRCAT member institutions. The authors also acknowledge the use of beamline 11-ID-C. We thank Evan Wegener for experimental assistance with the XAS.
Materials
| Name | Company | Catalog Number | Comments |
| 1 inch quartz tube reactor | Quartz Scientific | Processed by glass blower | |
| drying oven | Fisher Scientific | ||
| calcination Furnace | Thermo Sciencfic | ||
| clam-shell temperature programmed furnace | Applied Test System | Custom made | |
| propane dehydorgenation performance evaluation system | Homemade | ||
| gas chromatography | Hewlett-Packard | Model 7890 | |
| TEM grid | TedPella | 01824G | |
| pellet press | International Crystal Lab | 0012-8211 | |
| die set | International Crystal Lab | 0012-189 | |
| Linkam Sample Stage | Linkam Scientific | Model TS1500 | |
| copper nitrate trihydrgate | Sigma Aldrich | 61197 | |
| tetraammineplatinum nitrate | Sigma Aldrich | 278726 | |
| ammonia | Sigma Aldrich | 294993 | |
| silica | Sigma Aldrich | 236802 | |
| isopropyl alcohol | Sigma Aldrich | ||
| balance | Denver Instrument Company | A-160 | |
| spatulas | VWR | ||
| ceramic and glass evaporating dishes, beakers | VWR | ||
| heating plate | |||
| kimwipe papers | |||
| mortar and pestle | |||
| quartz wool | |||
| Swagelok tube fittings |
References
- Sattler, J. J., Ruiz-Martinez, J., Santillan-Jimenez, E., Weckhuysen, B. M. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 114 (20), 10613-10653 (2014).
- Childers, D. J., et al. Modifying structure-sensitive reactions by addition of Zn to Pd. J Catal. 318, 75-84 (2014).
- Gallagher, J. R., et al. Structural evolution of an intermetallic Pd-Zn catalyst selective for propane dehydrogenation. Phys. Chem. Chem. Phys. 17, 28144-28153 (2015).
- Wu, Z., et al. Pd-In intermetallic alloy nanoparticles: highly selective ethane dehydrogenation catalysts. Catal Sci Technol. 6 (18), 6965-6976 (2016).
- Siddiqi, G., Sun, P., Galvita, V., Bell, A. T. Catalyst performance of novel Pt/Mg (Ga)(Al) O catalysts for alkane dehydrogenation. J Catal. 274 (2), 200-206 (2010).
- Passos, F. B., Aranda, D. A., Schmal, M. Characterization and catalytic activity of bimetallic Pt-In/Al 2 O 3 and Pt-Sn/Al 2 O 3 catalysts. J Catal. 178 (2), 478-488 (1998).
- Virnovskaia, A., Morandi, S., Rytter, E., Ghiotti, G., Olsbye, U. Characterization of Pt, Sn/Mg (Al) O catalysts for light alkane dehydrogenation by FT-IR spectroscopy and catalytic measurements. J Phys Chem C. 111 (40), 14732-14742 (2007).
- Jablonski, E., Castro, A., Scelza, O., De Miguel, S. Effect of Ga addition to Pt/Al 2 O 3 on the activity, selectivity and deactivation in the propane dehydrogenation. Appl Catal A. 183 (1), 189-198 (1999).
- Galvita, V., Siddiqi, G., Sun, P., Bell, A. T. Ethane dehydrogenation on Pt/Mg (Al) O and PtSn/Mg (Al) O catalysts. J Catal. 271 (2), 209-219 (2010).
- Shen, J., Hill, J. M., Watwe, R. M., Spiewak, B. E., Dumesic, J. A. Microcalorimetric, infrared spectroscopic, and DFT studies of ethylene adsorption on Pt/SiO2 and Pt-Sn/SiO2 catalysts. J Phys Chem B. 103 (19), 3923-3934 (1999).
- Silvestre-Albero, J., et al. Microcalorimetric, reaction kinetics and DFT studies of Pt–Zn/X-zeolite for isobutane dehydrogenation. Catal Lett. 74 (1-2), 17-25 (2001).
- Sun, P., Siddiqi, G., Vining, W. C., Chi, M., Bell, A. T. Novel Pt/Mg (In)(Al) O catalysts for ethane and propane dehydrogenation. J Catal. 282 (1), 165-174 (2011).
- Sun, P., Siddiqi, G., Chi, M., Bell, A. T. Synthesis and characterization of a new catalyst Pt/Mg (Ga)(Al) O for alkane dehydrogenation. J Catal. 274 (2), 192-199 (2010).
- Okamoto, H. . Phase diagrams for binary alloys. Desk handbook. , (2000).
- Hamid, S. B. D. -. A., Lambert, D., Derouane, E. G. Dehydroisomerisation of n-butane over (Pt, Cu)/H-TON catalysts. Catal Today. 63 (2), 237-247 (2000).
- Veldurthi, S., Shin, C. -. H., Joo, O. -. S., Jung, K. -. D. Promotional effects of Cu on Pt/Al 2 O 3 and Pd/Al 2 O 3 catalysts during n-butane dehydrogenation. Catal Today. 185 (1), 88-93 (2012).
- Han, Z., et al. Propane dehydrogenation over Pt-Cu bimetallic catalysts: the nature of coke deposition and the role of copper. Nanoscale. 6 (17), 10000-10008 (2014).
- Komatsu, T., Tamura, A. Pt 3 Co and PtCu intermetallic compounds: promising catalysts for preferential oxidation of CO in excess hydrogen. J Catal. 258 (2), 306-314 (2008).
- Gallagher, J. R., et al. In situ diffraction of highly dispersed supported platinum nanoparticles. Catal Sci Technol. 4 (9), 3053-3063 (2014).
- Ma, Z., Wu, Z., Miller, J. T. Effect of Cu content on the bimetallic Pt-Cu catalysts for propane dehydrogenation. Catal Struct React. 3 (1-2), 43-53 (2017).
- Richards, R. . Surface and nanomolecular catalysis. , (2006).
- Jiao, L., Regalbuto, J. R. The synthesis of highly dispersed noble and base metals on silica via strong electrostatic adsorption: I. Amorphous silica. J Catal. 260 (2), 329-341 (2008).
- Miller, J. T., Schreier, M., Kropf, A. J., Regalbuto, J. R. A fundamental study of platinum tetraammine impregnation of silica: 2. The effect of method of preparation, loading, and calcination temperature on (reduced) particle size. J Catal. 225 (1), 203-212 (2004).
- Wei, H., et al. Selective hydrogenation of acrolein on supported silver catalysts: A kinetics study of particle size effects. J Catal. 298, 18-26 (2013).
- Ertl, G., Knözinger, H., Schüth, F., Weitkamp, J. . Handbook of heterogeneous catalysis: 8 volumes. , (2008).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved