A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
A Simple Fluorescence-based Reporter Assay to Identify Cellular Components Required for Ricin Toxin A Chain (RTA) Trafficking in Yeast
In This Article
Summary
In the manuscript, we describe the use of a yeast-based fluorescence reporter assay to identify cellular components involved in the trafficking and killing processes of the cytotoxic A subunit of the plant toxin ricin (RTA).
Abstract
Bacterial and plant A/B toxins exploit the natural trafficking pathways in eukaryotic cells to reach their intracellular target(s) in the cytosol and to ultimately kill. Such A/B toxins generally consist of an enzymatically active Asubunit (e.g., ricin toxin A (RTA)) and one or more cell binding Bsubunit(s), which are responsible for toxin binding to specific cell surface receptors. Our current knowledge of how A/B toxins are capable of efficiently intoxicating cells helped scientists to understand fundamental cellular mechanisms, like endocytosis and intracellular protein sorting in higher eukaryotic cells. From a medical point of view, it is likewise important to identify the major toxin trafficking routes to find adequate treatment solutions for patients or to eventually develop therapeutic toxin-based applications for cancer therapy.
Since genome-wide analyses of A/B toxin trafficking in mammalian cells is complex, time-consuming, and expensive, several studies on A/B toxin transport have been performed in the yeast model organism Saccharomyces cerevisiae. Despite being less complex, fundamental cellular processes in yeast and higher eukaryotic cells are similar and very often results obtained in yeast can be transferred to the mammalian situation.
Here, we describe a fast and easy to use reporter assay to analyze the intracellular trafficking of RTA in yeast. An essential advantage of the new assay is the opportunity to investigate not only RTA retro-translocation from the endoplasmic reticulum (ER) into the cytosol, but rather endocytosis and retrograde toxin transport from the plasma membrane into the ER. The assay makes use of a reporter plasmid that allows indirect measurement of RTA toxicity through fluorescence emission of the green fluorescent protein (GFP) after in vivo translation. Since RTA efficiently prevents the initiation of protein biosynthesis by 28S rRNA depurination, this assay allows the identification of host cell proteins involved in intracellular RTA transport through the detection of changes in fluorescence emission.
Introduction
Patients suffering from infections by toxin producing bacteria represent a severe medical and financial burden for each social health care system, in particular since efficient therapeutic treatments are still largely missing. To develop new therapeutic strategies, the complex intoxication mechanisms of medically relevant A/B toxins such as cholera toxin, Shiga toxin, or ricin need to be fully understood at the molecular level based on novel powerful assays that have to be implemented.
In recent years, several studies attempted to analyze A/B toxin transport in yeast and mammalian cells by using time-consuming and cost-intensive methods such as radioactive toxin labeling1,2 as well as siRNA-based screening approaches3. In some cases, toxin trafficking has been visualized in vivo by fluorescence microscopy after chemical and/or genetic coupling of individual toxin subunits with fluorophores, quantum dots, or fluorescent proteins4,5. Unfortunately, such modifications often lead to inactive and/or altered natural properties of the toxins. Another elegant way to indirectly answer a wide variety of scientific questions is the use of reporter systems based on enzymes such as lacZ, luciferase, or fluorescent proteins (e.g. GFP or Discosoma sp. red fluorescent protein (dsRed)).
In this manuscript, a simple protocol is described which identifies cellular components required for the intracellular transport of extracellularly applied RTA in S. cerevisiae. Thereby, a fluorescence-reporter plasmid containing an N-terminal ER-import signal followed by GFP acts as a protein biosynthesis sensor, which indirectly measures RTA-mediated protein translation inhibition by GFP fluorescence emission after in vivo translation6. In case that RTA endocytosis and/or intracellular trafficking is negatively (or positively) affected in a particular yeast deletion mutant compared to wild-type, this can be detected through an increase (or decrease) in GFP fluorescence emission6.
So far, all methods analyzing RTA transport in yeast cells were restricted to the ER-to-cytosol retro-translocation process of RTA. In such an artificial system, RTA containing an ER import signal is expressed from an inducible promoter resulting in a suicidal phenotype1,7. Although the cell binding B-subunit of ricin is likewise missing in the experimental setup described in this manuscript and, thus, does not fully represent the natural situation of ricin holotoxin intoxication8, toxin transport from the plasma membrane through the Golgi apparatus to the ER can be closely mimicked with this novel assay. Interestingly, the preliminary results obtained in the pilot study indicate that the trafficking pathways used by RTA reveal striking similarities with the intoxication route of ricin holotoxin.
In summary, the described method can be used to determine the specific role of selected cellular proteins in RTA endocytosis and trafficking in yeast. Furthermore, this experimental setup might be easily adapted to other ribosome inactivating toxins produced and secreted from various yeast and bacterial species such as zymocin or Shiga toxin.
Protocol
NOTE: An overview of the general experimental workflow is depicted in Figure 1.
Caution: RTA is highly toxic for humans. Safety lab permission S2 (biosafety level 2 equivalent) is needed. Please wear gloves during the entire experiment.
1. Heterologous Expression of His-tagged RTA in Escherichia coli
- Transform E. coli cells with the expression plasmid pET24-RTA(His)6 or the empty vector pET24a(+) using standard electroporation protocols9,10. Cells containing the empty plasmid serve as a negative control.
- After selection of positive clones on kanamycin-containing (100 µg/mL) LB plates, inoculate cells containing the RTA expression plasmid or the empty vector in 5 mL LBkan medium (LB medium with 100 µg/mL of kanamycin) and incubate at 37 °C and 220 rpm for 24 h.
- Supplement 1 L LBkan medium with 5 mL pre-culture and incubate cells at 37 °C and 220 rpm until cells have reached an OD600 of 0.8-1.0 (approximately 3-4 h). Thereafter, reduce culture temperature to 28 °C and prepare a 1 M of isopropyl-β-D-1-thiogalactopyranoside (IPTG) stock solution in H2O.
- Induce RTA expression of the E. coli by adding IPTG to a final concentration of 1 mM.
- After 3.5 h at 28 °C and 220 rpm, harvest cells by centrifugation at 10,000 x g and 4 °C for 10 min, wash the pellet two times with 5 mL of binding buffer (500 mM NaCl, 10 mM imidazole, and 20 mM KH2PO4, pH = 7.4) at 10,000 x g and 4 °C for 10 min, and resuspend pellet in 5 mL binding buffer.
NOTE: Protocol can be paused at this stage and cells can be stored at 80 °C for several days.
2. Purification of His-tagged RTA via Affinity Chromatography
- Sonicate cells on ice using the following protocol: 15 s pulse (20 microns), 30 s pause. Repeat this step five times.
- Centrifuge cell lysate at 21,000 x g and 4 °C for 15 min and filter supernatant using a sterile syringe filter system (0.2 µm pore size).
NOTE: Cell pellets of successfully sonicated samples show transparent borders. - Use an automated purification system equipped with a 5 mL Ni2+-based affinity column to purify the His-tagged RTA fraction from the sterile-filtered E. coli supernatant. In general, use an elution speed of 1 mL/min and cool the whole purification system to prevent non-efficient toxin binding and loss of toxin activity.
NOTE: Parameters for efficient RTA purification are listed in Table 1. See also Becker et al. for further information9.- Briefly, equilibrate the affinity column with 20 mL of binding buffer to remove the storage buffer. Apply sterile-filtered supernatant onto the affinity column using a syringe.
- Wash the column with 25-35 mL of binding buffer to remove the unbound proteins from the column. Perform the washing step until UV absorbance at 280 nm is close to the initial UV value.
- Elute bound RTA fraction in 20-35 mL of elution buffer (500 mM imidazole, 500 mM NaCl, 20 mM KH2PO4, pH = 7.2) and keep the sample on ice (Figure 2A and Figure 2B).
NOTE: Elution of the RTA fraction is marked by an increase in UV absorption. Please note to exclusively collect this fraction to prevent contamination with nonspecific bound proteins. - Use 20 µL of eluted samples to perform Coomassie Blue staining11 or Western blot analysis12,13 (Optional). Use primary anti-His antibodies (1:1,000) and secondary anti-mouse-IgG-HRP (1:10,000) to detect RTA and verify sample purity (Figure 3).
- Desalt eluted RTA fraction on a 5 mL desalting column and equilibrate sample in 0.8 M sorbitol.
- Replace the affinity column of the purification system by the desalting column and first equilibrate the column with 20 mL of 0.8 M sorbitol.
- Apply the eluted RTA fraction to the column via a syringe. Wash the column with 100 mL of 0.8 M sorbitol and elute the desalted RTA fraction in a 15 mL tube as soon as the UV absorption starts to increase (Figure 2C).
- Store the eluted RTA fraction at 4 °C.
NOTE: Directly stop RTA fraction sampling when conductance increases to avoid salt contamination. Parameters for the desalting procedure are listed in Table 1.
- Concentrate eluted RTA fraction at 10,000 x g and 4 °C for 30-180 min using a 10 kDa cut-off spin column to a final volume of 1-2 mL and store sample at 4 °C for 3-4 weeks.
Caution: Do not freeze the sample since freezing leads to a complete loss of RTA activity. - Determine protein concentration using a conventional protein determination kit. Protein concentration should be in the range of 1-1.5 mg/mL.
NOTE: RTA tends to precipitate if the protein concentration is too high (>5 mg/mL).
3. Yeast Transformation and Cell Wall Removal
- Transform wild-type or selected yeast deletion mutants with the GFP reporter plasmid pRS315-K28SP-GFP6 using standard lithium acetate transformation methods14. Incubate cells on leucine drop-out (d/o) glucose plates (2% glucose, 1.5% agar, 0.5% ammonium sulfate, 0.17% Yeast Nitrogen Base (YNB), and 0.087% d/o mix without leucine) at 30 °C for 2-3 days for positive clone selection.
- Pick 3 different yeast clones from each plate and inoculate the clones in 100 mL of leucine d/o raffinose medium (2% raffinose, 0.5% ammonium sulfate, 0.17% YNB, and 0.087% d/o mix without leucine) at 220 rpm and 30 °C to OD600 = 1.0-2.0 (2-4 x 107 cells/mL).
NOTE: Cell growth of the different yeast deletion strains is different. Monitor OD600 via a spectrophotometer. - To calculate OD600 values, dilute samples to OD600 = 0.1-0.3 (1:5 to 1:10 dilutions) and measure OD600 in a spectrophotometer. As reference, use H2O supplemented with the corresponding amount of leucine d/o raffionose medium. Finally, resuspend OD600 = 125 in 5 mL of sterile water (5 x 108 cells/mL).
- Centrifuge cells at 25 °C for 5 min at 10,000 x g, wash cells twice with 5 mL sterile water, and resuspend cells in 50 mL spheroplasting buffer (0.8 M sorbitol, 10 mM Tris-HCl (pH 7.5), 10 mM CaCl2, 2 mM dithiothreitol (DTT), and 200 µg/mL lytic enzyme).
- Incubate the 50 mL culture at 100 rpm and 30 °C for 90 min. Optional: Monitor spheroplast formation every 15 min by phase contrast microscopy. Under the microscope, cells should have a dark translucent gray color. Bright (refractile) cells are insufficiently digested, whereas ghosts (pale gray cells with little, if any, internal structure) are over-digested.
- Check spheroplast preparation efficacy for each sample.
- After finishing step 3.5, mix 4 µL of the spheroplasted 50 mL culture with 496 µL spheroplasting buffer (approximately 2 × 106 spheroplasts) and centrifuge for 10 min at 400 x g.
- Resuspend pellet in 10 mL H2O distilled, vortex sample for 30 s, and plate out 10 µL of the sample on leucine d/o glucose plates (2% glucose, 1.5% agar, 0.5% ammonium sulfate, 0.17% YNB, and 0.087% d/o mix without leucine).
- Incubate cells for 3 days at 30 °C. Count the total number of grown cell colonies on the plate. For data evaluation, use only samples with efficiency higher than 98% (total cell colony number <40 colonies/plate).
- Centrifuge cells from step 3.5 at 400 x g and 4 °C for 10 min and wash cells twice with 5 mL stabilized leucine d/o raffinose medium (0.8 M sorbitol, 2% raffinose, 0.5% ammonium sulfate, 0.17% YNB, and 0.087% drop-out mix without leucine). Resuspend cells in 5 mL stabilized leucine d/o raffinose medium (1 x 108 cells/mL) and directly use cells for the GFP reporter assay measurements (Section 4).
4. GFP Reporter Assay Measurement in 96-Well Plates
- Seed out the yeast cell spheroplasts obtained in step 3.7 into 96-well plates (200 µL/well).
- Add 70 µL stabilized leucine d/o raffinose medium containing negative control (eluate of a Ni2+-affinity purified cell lysate from IPTG-induced E. coli cells expressing the empty vector) or purified RTA in a final RTA concentration of 5 µM (corresponding to 160 g/L RTA) in each well.
- Immediately add 30 µL stabilized galactose solution (30% galactose, 0.8 M sorbitol) to induce GFP expression and subsequently start the measurement.
NOTE: Perform at least 3 technical replicates per experiment and 3 biological replicates per yeast strain. - After finishing sample preparation (steps 4.1-4.3), put the 96-well plate in a fluorescence reader and start the measurement. Use the 475/509 nm filter set required for GFP fluorescence detection. Perform measurements at 30 °C, 120 rpm, and with a shaking diameter of 1 mm over a time window of 20 h (measuring intervals of 10 min).
NOTE: The GFP filter set is normally available in all reader systems. Temperature, time window, measuring intervals, and RTA concentration can be adjusted for own needs. Representative results are shown in Figure 4. - Optional: Use additional internal controls in the measurement for quality control. Prepare a negative control by adding 30 µL stabilized leucine d/o raffinose medium instead of 30% galactose (no GFP induction). In addition, add 70 µL of 0.8 M sorbitol stabilized G418 solution (300 µg/mL). The protein translation inhibitor G418 serves as positive control for protein inhibition as it prevents GFP expression in yeast.
- Calculate relative GFP fluorescence in percent for the 20 h time point according to the following equation (see Figure 4A):
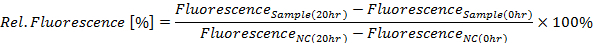 Where NC is the negative control
Where NC is the negative control
- Alternatively, determine the relative GFP fluorescence for each measuring point (in this case 10 min, see also step 4.4) using the above equation. As shown in Figure 4B, create a graph by blotting the GFP fluorescence intensities (y-axis) over time (x-axis).
Results
The general workflow of the protocol described in this manuscript is illustrated in Figure 1, roughly summarizing the single steps for successful RTA purification and the subsequent GFP reporter assay experiment. A more detailed description of each individual step can be found in the protocol. Figure 2 illustrates the expected result of a successful RTA purification by affinity chromatography (Figure 2A
Discussion
When performing the above protocol, we recommend the following suggestions to achieve a successful outcome of the experiment.
For heterologous protein expression, it is important to not exceed the IPTG concentration of 1 mM. IPTG concentrations >1 mM inhibit promoter-induced RTA expression and lead to lower toxin yields. Furthermore, cells should not be cultivated at temperatures higher than 28 °C to prevent inclusion body formation, inefficient folding, and toxin inactivation. RTA ex...
Disclosures
The authors have nothing to disclose.
Acknowledgements
Parts of this study were kindly supported by a grant from the Deutsche Forschungsgemeinschaft (SFB 1027, A6).
Materials
| Name | Company | Catalog Number | Comments |
| Bacterial and yeast strains | |||
| E. coli BL21 DE3(Gold) | Aligent Technologies | 230130 | |
| S. cerevisiae BY4742 | Euroscarf | Y10000 | |
| S. cerevisiae BY4742 deletion mutants | Dharmacon | YSC1054 | whole collection |
| Name | Company | Catalog Number | Comments |
| Plasmids used in this protocol | |||
| pET24a(+) (Novagen) | Millipore | 69772-3 | |
| pET-RTA(His6) | Becker et al. (2016)3 | ||
| Name | Company | Catalog Number | Comments |
| Reagents | |||
| Zymolyase 20T | USBio | Z1000.250 | lytic enzyme for cell wall removal |
| LB broth medium | Thermo Scientific | 10855021 | 15 g agar was added for plate production |
| YNB | Thermo Scientific | DF0335-15-9 | |
| Ammonium sulfate | Sigma-Aldrich | A4418-100G | |
| Yeast drop-out mix supplemts without leucine | Sigma-Aldrich | Y1376-20G | |
| Agar | Sigma-Aldrich | 05040-100G | |
| D-glucose | Sigma-Aldrich | G8270-100G | |
| DTT | Sigma-Aldrich | 10197777001 | |
| D-raffinose | Sigma-Aldrich | 83400-25G | |
| D-sorbitol | Sigma-Aldrich | S1876-1KG | |
| D-galactose | Sigma-Aldrich | G0750-10MG | |
| G418 | Thermo Scientific | 11811031 | |
| IPTG | Sigma-Aldrich | I6758-1G | |
| Imidazole | Roth | 3899.1 | |
| PAGE ruler prestained | Fermentas | 26616 | protein ladder used for Western analysis |
| Name | Company | Catalog Number | Comments |
| Material for RTA purification, desalting and reader measurements | |||
| Spectrophotometer Ultrospec 2100 pro | Amersham | ||
| Soniprep 150 | MSE | old model, other models available | |
| Fluoroskan Ascent | Thermo Scientific | 5210470 | old model, not available anymore |
| ÄKTAPurifier | Thermo Scientific | 28406266 | Product is discontinued and replaced |
| HisTRAP HP column | GE Healthcare | 17-5248-02 | |
| HiTRAP desalting column | GE Healthcare | 11-0003-29 | |
| Midisart sterile filter | Sartorius | 16534K | 0.2 µm pore size |
| BCA protein assay kit | Pierce | 23225 | |
| 660 nm assay kit | Thermo Scientific | 22660 | |
| 96 well plates | Thermo Scientific | 260860 | |
| Name | Company | Catalog Number | Comments |
| Antibodies (optional) | |||
| Anti-Tetra-His | Qiagen | 34670 | primary antibody; 1:1,000 dilution |
| Anti-mouse-HRP | Sigma-Aldrich | A9044-2ML | secondary antibody, 1:10,000 dilution |
References
- Li, S., et al. Folding-competent and folding-defective forms of ricin A chain have different fates after retrotranslocation from the endoplasmic reticulum. Mol Biol Cell. 21 (15), 2543-2554 (2010).
- Li, S., Spooner, R. A., Hampton, R. Y., Lord, J. M., Roberts, L. M. Cytosolic entry of Shiga-like toxin a chain from the yeast endoplasmic reticulum requires catalytically active Hrd1p. PLoS One. 7 (7), e41119 (2012).
- Moreau, D., et al. Genome-wide RNAi screens identify genes required for Ricin and PE intoxications. Dev Cell. 21 (2), 231-244 (2011).
- Giepmans, B. N., Adams, S. R., Ellisman, M. H., Tsien, R. Y. The fluorescent toolbox for assessing protein location and function. Science. 312 (5771), 217-224 (2006).
- Majoul, I. V., Bastiaens, P. I., Soling, H. D. Transport of an external Lys-Asp-Glu-Leu (KDEL) protein from the plasma membrane to the endoplasmic reticulum: studies with cholera toxin in Vero cells. J Cell Biol. 133 (4), 777-789 (1996).
- Becker, B., Schnoder, T., Schmitt, M. J. Yeast Reporter Assay to Identify Cellular Components of Ricin Toxin A Chain Trafficking. Toxins (Basel). 8 (12), (2016).
- Li, X. P., Baricevic, M., Saidasan, H., Tumer, N. E. Ribosome depurination is not sufficient for ricin-mediated cell death in Saccharomyces cerevisiae. Infect Immun. 75 (1), 417-428 (2007).
- Lord, J. M., Roberts, L. M., Robertus, J. D. Ricin: structure, mode of action, and some current applications. Faseb J. 8 (2), 201-208 (1994).
- Becker, B., Schmitt, M. J. Adapting yeast as model to study ricin toxin a uptake and trafficking. Toxins (Basel). 3 (7), 834-847 (2011).
- Seidman, C. E., Struhl, K., Sheen, J., Jessen, T. Introduction of plasmid DNA into cells. Curr Protoc Mol Biol. , (2001).
- Brunelle, J. L., Green, R. Coomassie blue staining. Methods Enzymol. 541, 161-167 (2014).
- Shapiro, A. L., Vinuela, E., Maizel, J. V. Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 28 (5), 815-820 (1967).
- Towbin, H., Staehelin, T., Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. 1979. Biotechnology. 24, 145-149 (1992).
- Ito, H., Fukuda, Y., Murata, K., Kimura, A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 153 (1), 163-168 (1983).
- Vitetta, E. S., Yen, N. Expression and functional properties of genetically engineered ricin B chain lacking galactose-binding activity. Biochim Biophys Acta. 1049 (2), 151-157 (1990).
- Wales, R., Roberts, L. M., Lord, J. M. Addition of an endoplasmic reticulum retrieval sequence to ricin A chain significantly increases its cytotoxicity to mammalian cells. J Biol Chem. 268 (32), 23986-23990 (1993).
- Breslow, D. K., et al. A comprehensive strategy enabling high-resolution functional analysis of the yeast genome. Nat Methods. 5 (8), 711-718 (2008).
- Jablonowski, D., Schaffrath, R. Zymocin, a composite chitinase and tRNase killer toxin from yeast. Biochem Soc Trans. 35 (Pt 6), 1533-1537 (2007).
- Jablonowski, D., Schaffrath, R. Saccharomyces cerevisiae RNA polymerase II is affected by Kluyveromyces lactis zymocin. J Biol Chem. 277 (29), 26276-26280 (2002).
- Jablonowski, D., Frohloff, F., Fichtner, L., Stark, M. J., Schaffrath, R. Kluyveromyces lactis zymocin mode of action is linked to RNA polymerase II function via Elongator. Mol Microbiol. 42 (4), 1095-1105 (2001).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved