A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Measuring Deformability and Red Cell Heterogeneity in Blood by Ektacytometry
* These authors contributed equally
In This Article
Summary
Here we present techniques to measure red cell deformability and cellular heterogeneity by ektacytometry. These techniques are applicable to general investigations of red cell deformability and specific investigations of blood diseases characterized by the presence of both rigid and deformable red cells in circulation, such as sickle cell anemia.
Abstract
Decreased red cell deformability is characteristic of several disorders. In some cases, the extent of defective deformability can predict severity of disease or occurrence of serious complications. Ektacytometry uses laser diffraction viscometry to measure the deformability of red blood cells subject to either increasing shear stress or an osmotic gradient at a constant value of applied shear stress. However, direct deformability measurements are difficult to interpret when measuring heterogenous blood that is characterized by the presence of both rigid and deformable red cells. This is due to the inability of rigid cells to properly align in response to shear stress and results in a distorted diffraction pattern marked by an exaggerated decrease in apparent deformability. Measurement of the degree of distortion provides an indicator of the heterogeneity of the erythrocytes in blood. In sickle cell anemia, this is correlated with the percentage of rigid cells, which reflects the hemoglobin concentration and hemoglobin composition of the erythrocytes. In addition to measuring deformability, osmotic gradient ektacytometry provides information about the osmotic fragility and hydration status of erythrocytes. These parameters also reflect the hemoglobin composition of red blood cells from sickle cell patients. Ektacytometry measures deformability in populations of red cells and does not, therefore, provide information on the deformability or mechanical properties of individual erythrocytes. Regardless, the goal of the techniques described herein is to provide a convenient and reliable method for measuring the deformability and cellular heterogeneity of blood. These techniques may be useful for monitoring temporal changes, as well as disease progression and response to therapeutic intervention in several disorders. Sickle cell anemia is one well-characterized example. Other potential disorders where measurements of red cell deformability and/or heterogeneity are of interest include blood storage, diabetes, Plasmodium infection, iron deficiency, and the hemolytic anemias due to membrane defects.
Introduction
Ektacytometry provides a convenient measure of red cell deformability in response to alterations in shear stress (measured in pascals (Pa)) or suspending medium osmolality. Pertinent parameters of red cell deformability include the maximum elongation index (EI Max), a measure of the maximum deformability of a red cell in response to increasing shear stress, and shear stress ½ (SS ½), the shear stress required to achieve half maximal deformability.1 Osmotic gradient ektacytometry has several informative parameters. These include the elongation index minimum (EI Min), a measure of surface-to-volume ratio and the osmolality at which it occurs (O Min), which is a measure of osmotic fragility. EI Max and the osmolality at which it occurs (O (EI Max)) provide information on membrane flexibility and cell surface area. Half maximal elongation in the hypertonic arm of the osmotic gradient is represented by EI hyper. EI hyper and the osmolality at which it occurs, O hyper, provide information about the intracellular viscosity of the red cell which is determined by hemoglobin concentration.2,3 Measuring deformability in heterogenous blood is complicated by the fact that rigid cells, such as sickled red blood cells, do not properly align with the direction of flow such as deformable cells in response to increasing shear stress. Rather than producing a characteristic elliptical diffraction image, rigid cells produce a spherical pattern which results in a diamond-shaped diffraction pattern when overlaid on the ellipse produced by deformable cells.4,5,6 The spherical pattern has been shown to correspond to irreversibly sickled cells by performing ektacytometry on isolated fractions of cells following density centrifugation.6 The elongation index calculation includes measures of both the long and short axis of the ellipse; a diamond shape therefore produces an apparent decrease in elongation by increasing the width of the short axis.7 It has been previously shown that the degree of diffraction pattern distortion is correlated with both the percentage of sickle hemoglobin (HbS) and the percentage of sickled cells in the blood from patients with sickle cell anemia.5 The degree of diffraction pattern distortion can be obtained by complex mathematical analyses.8 It can also be obtained by adjusting the opening of the camera aperture on the ektacytometer or the grey level of the fitting software to alter the diffraction pattern height.5 However, details regarding how to adjust the grey level are not well defined and the camera aperture is not readily accessible on the latest generation of the commercially available ektacytometer. To circumvent these issues, the easily accessible camera gain can be used to adjust diffraction pattern heights.9 Using this method to estimate cellular heterogeneity, the degree of diffraction pattern distortion can be correlated with the percentage of fetal hemoglobin in the blood of patients with sickle cell anemia.10 Several osmotic gradient ektacytometry parameters are likewise correlated with the percentage of fetal or sickle hemoglobin in blood from patients with sickle cell anemia. Diffraction pattern distortion correlations likely reflect the contribution of hemoglobin composition to the percentage of rigid, non-deformable cells. Of additional interest, the entire osmotic gradient ektacytometry profile undergoes biphasic changes that correspond to the percentage of dense cells in circulation during sickle cell crisis.11
Ektacytometry is likewise useful in the study of several other disorders. Osmotic gradient ektacytometry is diagnostic for the inherited red cell membrane disorders, such as hereditary spherocytosis, hereditary elliptocytosis and hereditary pyropoikilocytosis.3,12,13,14 Decreased deformability occurs in iron deficiency.15 Characterization of the "storage lesion" of blood has employed ektacytometry and future studies investigating both the nature of the lesion and interventions to prevent its formation during the storage of banked blood are likely to benefit from the techniques presented here.16 Decreased red cell deformability has also been correlated with microvascular disease in diabetes.17 Recent studies linking hyperglycemia, red cell ascorbate concentrations and osmotic fragility suggest these factors may be important in the development of microvascular disease.18 Ektacytometry studies are currently underway to investigate this hypothesis (Parrow and Levine, unpublished data). Blood stage malarial infection is another interesting avenue of red cell deformability investigations. Cellular deformability of Plasmodium falciparum infected red blood cells decreases dramatically during the 48 hours of intracellular maturation of the parasite from ring stage to schizont stage. Evidence indicates that this decreased deformability is reversed upon maturation of the parasite. The reversal coincides with release of infected red cells into the circulation. Decreased deformability is thought to be mediated by Plasmodium proteins that promote sequestration of the red cell.19 These studies represent a small sampling of clinically important conditions where measuring erythrocyte deformability and osmotic gradient parameters are relevant. Several additional areas of study exist.
Alternative techniques for measuring red cell deformability include optical tweezers (also known as laser traps) which use the physical properties of photons to stretch single red cells in one or more directions.20 This technique has the advantage of measuring the deformability of single erythrocytes, but some uncertainty in force calibration has produced considerable variability across studies 21 and data analysis can be labor-intensive unless automated.22 Micropipette aspiration, which uses negative pressure to aspirate an erythrocyte into a micropipette, has also been used to measure deformability of red cells.7,23 Multiple measurements, such as the pressure required to aspirate the red cell, are possible with each measure defining different characteristics of the red cell.23 Atomic force microscopy is a high resolution technique that measures membrane stiffness by quantifying laser beam deflection as an indicator of cantilever deflection along the surface of a red cell.24 These techniques provide information about individual erythrocytes, are not easily adapted to measure changes in populations of red blood cells, and, in general, require considerable technical expertise.
The desire to sample both individual and populations of cells simultaneously has led to advances in automation and the development of microfluidics and array-based methods. Like ektacytometry, rheoscopy measures deformability as a function of shear stress but images are acquired directly via microscope.25 For higher through-put analyses, automated cell imaging has been employed to produce deformability distributions using the rheoscope.26 Cellular heterogeneity can be quantified by this method if data from a healthy control subject are available.27 Microfluidics techniques also allow for high through-put analyses of single cells; multiple designs using adaptations of filtration,28 cell transit analyzers,29 which measures the time required for an erythrocyte flow through a micropore, and alternatives that measure the pressure required for erythrocyte transit rather than time 30 have been developed. Another platform for high through-put analysis of individual cells is the single cell microchamber array chip, which has the additional advantage of allowing for downstream fluorescence-based characterization of the cells.31 Although each of these techniques is potentially useful and may be superior for particular applications, the comparative advantages of ektacytometry includes sensitivity, ease of use, and precision.32 The latest generation of commercially available ektacytometers also possess considerable versatility in the number of assays that can be performed.
Access restricted. Please log in or start a trial to view this content.
Protocol
All subjects in this study gave written informed consent in accordance with the Declaration of Helsinki and the National Institutes of Health Institutional Review Board approved protocols.
1. Turning on the ektacytometer
- Connect the tubing from the cleaning solution to the low and high osmolar polyvinylpyrrolidone (PVP) solutions. Be careful to connect the 0 osmolar tube to the low osmolar solution and the 500 osmolar tube to the high osmolar solution.
Note: The low osmolar PVP solution should have an osmolality between 35 and 55 milliosmoles per kilogram (mOsm/kg), a pH of 7.25-7.45 at 25 °C and a viscosity between 27.0 and 33.0 centipoise (cP) at 37.0 ± 0.5 °C. The high osmolar PVP solution should have an osmolality between 764 and 804 mOsm/kg, a pH of 7.25-7.45 at 25 °C and a viscosity measure of 27.0-33.0 cP at 37.0 ° 0.5 °C. - Ensure that the bob is lowered completely into the cup. Launch the software and prime the machine (Hardware Check | Instrument IO). Allow the instrument to complete the priming cycle. Once the cycle is complete, lift the bob out of the cup and completely dry the bob and the cup with a low lint cleaning tissue.
Note: Residual water will lyse red cells, producing interference.
2. Measuring deformability as a function of increasing shear stress
- Obtain blood (less than 1 mL is adequate to perform these techniques with replicates) in vial containing an appropriate anticoagulant.
Note: EDTA is preferred over heparin because it has less influence over hemorheological parameters.33 Keep blood at room temperature if measurements are performed within 6 hours of blood draw. - Gently mix whole blood sample before testing by inverting the vial several times. Add 25 µL of whole blood to 5 mL of iso-osmolar PVP solution by pipetting, cap the vial and mix gently by inverting several times. The iso-osmolar PVP solution should have an osmolality between 284 and 304 mOsm/kg, a pH of 7.3-7.4 at 25 °C and a viscosity of 27.0-33.0 cP at 37.0 ± 0.5 °C.
- On the software choose deformability from the main menu. Create a new analysis and add experimental details (Deformability | Add desired details | Okay).
- Lift lid to ektacytometer, verify that the bob is fully lowered into cup and the cup is turning.
- Add 1 mL of PVP blood solution into the space between the cup and bob by pipetting.
- Lift the bob slightly to bring samples down. Wait until all bubbles have moved out of the solution, then close the lid to the ektacytometer. If needed, press the aspiration button to help remove bubbles.
- Adjust the gain to 200 by moving the arrow along the scroll bar on the software (see note). When the temperature is stable at 37 °C and the diffraction image is stable, press start (Start).
Note: For many studies, a good diffraction image can be obtained from healthy blood (hemoglobin concentration > 12.0 g/dL, mean corpuscular volume of 80-96 fL and mean corpuscular hemoglobin concentrations of 33-36 g/dL) with the camera gain set to 200. For studies of blood from sickle cell anemia, adjusting the camera gain to generate a 4.5 cm diffraction image has been suggested as the default setting to allow comparison of results across studies and laboratories.9 - Observe diffraction patterns as data acquisition progresses to ensure that they remain circular, elliptical or diamond shaped. When data acquisition is complete, save or print the report (File | Save or File | Print). EI Max and SS ½ values will be reported automatically, along with elongation indices corresponding to user-specified or default shear stresses. Data will also be saved automatically by the software.
- At the end, press the clean option on the dialog box on the computer monitor (Clean). After the sample is aspirated, rinse the space between the cup and bob by squirting deionized water into the space while the instrument remains in the clean cycle. Once the clean cycle is complete, lift the bob out of the cup and completely dry the bob and the cup with a low lint cleaning tissue (critical: Residual water will lyse red cells, producing interference).
- Click on the Main Menu button on the software to return to the main page (Main Menu).
3. Measuring cellular heterogeneity
- Gently mix whole blood sample before testing by inverting the vial several times. Pipet 25 µL of whole blood to a new 5 mL vial of iso-osmolar PVP solution, cap the vial and mix gently by inverting until the mixture is homogenous.
- Choose deformability from the main menu. Create a new analysis and add experimental details (Deformability | Add desired details | Okay).
- Lift lid to ektacytometer, verify that the bob is fully lowered into cup and the cup is turning.
- Pipet 1 mL of the PVP blood solution into the space between the cup and bob.
- Wait until all bubbles have moved out of the solution, then close the lid to the ektacytometer.
- Ensure that a stable diffraction image is present on the screen. Adjust the camera gain by moving the arrow along the scrollbar on the software until it produces a 3.8 cm diffraction height. Use a ruler to verify the height of the image on the computer screen.
- When the temperature is stable at 37 °C and the diffraction image is stable, press start (Start).
- Observe diffraction patterns as data acquisition progresses to ensure that they remain circular, elliptical or diamond-shaped. When data acquisition is complete, save or print the report (File | Save or File | Print).
- At the end, press the clean option on the dialog box on the computer monitor (Clean). After the sample is aspirated, rinse the space between the cup and bob by squirting deionized water from a squirt bottle into it while the instrument remains in the clean cycle. Once the clean cycle is complete, lift the bob out of the cup and completely dry the bob and the cup with a low lint cleaning tissue (critical: Residual water will lyse red cells, producing interference).
- Repeat steps 2.1-2.5 and adjust camera gain to obtain a 4.5 cm diffraction pattern height (step 2.2).
- Repeat steps 2.1-2.5 and adjust camera gain to obtain a 5.4 cm diffraction pattern height (step 2.2).
- At the end, click on the main menu button to return to the main page of the software (Main Menu).
- To determine the degree of diffraction pattern distortion based on EI Max as a percentage, use the following equation (the same equation can be performed with the data from the 4.5 cm diffraction pattern height if desired):
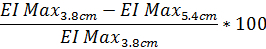
- Similarly, to determine the degree of diffraction pattern distortion based on SS1/2 use the same equation with the reported SS1/2 value:
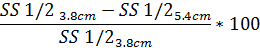
4. Osmotic gradient ektacytometry
- Obtain sample as described in 1.1. Gently mix whole blood sample before testing by inverting several times. Add 250 µL of whole blood to 5 mL iso-osmolar PVP vial by pipetting, cap the vial and mix gently by inverting until the mixture is homogenous.
- Choose osmoscan from the main menu (Osmoscan). Place vial containing the blood PVP solution underneath the needle on the left-hand side of the machine. Lower the needle until it touches the bottom of the vial. Make sure tubing is properly connected to the low and high osmolar solutions for gradient production. Close the lid to the ektacytometer and open the door on the lower half so that you can watch the blood enter the tubing.
- Press new analysis and type in experimental details (Osmoscan | New Analysis | Enter desired details | Okay). Adjust the camera gain to 200 by moving the arrow controlling it on the software and allow the machine run until blood is seen entering the cup from the tubing beneath the instrument.
- Once the blood has entered the cup and a stable diffraction pattern image is on the computer screen, begin data acquisition by pressing the start now button on the dialog box (Start Now).
- Allow the ektacytometer to acquire data up to approximately 500 mOsm/kg, then stop the instrument. Save or print report (File | Save or File | Print). Data will also save automatically.
- Remove the old PVP-blood vial. Replace it with a clean vial containing deionized water. Place it beneath the needle, bring the needle down so that it touches the bottom of the vial and press the rinse button in the dialog box to rinse the gradient system (Rinse).
- Once the rinse is complete, press the clean option on the dialog box on the computer monitor (Clean). Once the clean cycle is complete, lift the bob out of the cup and completely dry the bob and the cup with a low lint cleaning tissue (critical: Residual water will lyse red cells, producing interference).
Note: The osmoscan report provides elongation indices across the osmotic gradient. EI Min, O (EI Min), EI Max, O (EI Max), EI hyper and O hyper are generated automatically and included in the report. The range of osmotic gradient ektacytometry parameters obtained from blood from 9 healthy volunteers is: EI Min 0.12-0.196 arbitrary units (a.u.); O Min 117-144 mOsm/kg; EI Max 0.551-0.573 a.u.; O (EI Max) 272-312 mOsm/kg; EI hyper 0.278-0.286; O Hyper 454-505 mOsm/kg.
5. Turning off the ektacytometer
- Clean the instrument properly before it is shut down.
- To do this, connect the tubing from the low and high osmolar solutions to the y-adapter leading to the cleaning solution. Place a vial containing cleaning solution below the needle on the left-hand side of the machine and lower the needle until it touches the bottom of the vial. Ensure that the bob is lowered completely into the cup.
- Close the software, and press start on the end of day clean dialog box on the computer monitor (Close | Start). Allow the instrument to cycle through cleaning completely.
- Disconnect the tubing to the waste bottle and remove it from the instrument to discard waste. Dry the bob completely. Turn the machine off.
Access restricted. Please log in or start a trial to view this content.
Results
The ektacytometry results described in this manuscript can be used to measure red cell deformability in any condition. A schematic of the general set up of an ektacytometer is shown in Figure 1. Homogeneous populations of erythrocytes will produce an elliptical diffraction pattern in response to increasing shear stress that can be used to calculate the elongation index as shown in Figure 2. Diffraction pattern distortion occurs i...
Access restricted. Please log in or start a trial to view this content.
Discussion
The ektacytometry techniques described are straightforward and well automated, ensuring valid and reproducible results. Nonetheless, some critical steps exist. Proper temperature control of the blood is important. Storage at room temperature for more than eight hours may affect SS ½ values.34 Ensuring that the temperature of the machine is stable at 37 °C is also important, as viscosity of the suspending medium is temperature dependent. Blood should be fully oxygenated to avoid decreased...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institutes of Diabetes, Digestive and Kidney Diseases and the National Heart, Lung and Blood Institute of the National Institutes of Health. The opinions expressed herein are the sole responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| LoRRca MaxSis standard version | Mechatronics | LORC109000 | |
| LoRRca MaxSis Osmoscan | Mechatronics | LORC109001 | |
| Polyvinylpyrrolidone solution (PVP) 0mOsm | Mechatronics | QRR030910 | |
| Polyvinylpyrrolidone solution (PVP) 500mOsm | Mechatronics | QRR030930 | |
| Polyvinylpyrrolidone solution (PVP) 5mL vials | Mechatronics | QRR030901 | |
| X clean | Mechatronics | QRR010946 | |
| P1000 | MilliporeSigma | Z646555 | |
| P200 | MilliporeSigma | Z646547 | |
| P200 filter tips | MidSci | AV200-H | |
| P1250 filter tips | MidSci | AV1250-H | |
| Kimwipes | MidSci | 8091 | |
| 1.5 mL eppendorf tubes | MidSci | AVSS1700 | |
| 15 mL conical vial | MidSci | C15R |
References
- Bessis, M., Mohandas, N., Feo, C. Automated ektacytometry: a new method of measuring red cell deformability and red cell indices. Blood Cells. 6 (3), 315-327 (1980).
- Clark, M. R., Mohandas, N., Shohet, S. B. Osmotic gradient ektacytometry: comprehensive characterization of red cell volume and surface maintenance. Blood. 61 (5), 899-910 (1983).
- Da Costa, L., et al. Diagnostic tool for red blood cell membrane disorders: Assessment of a new generation ektacytometer. Blood Cells Mol Dis. 56 (1), 9-22 (2016).
- Clark, M. R., Mohandas, N., Shohet, S. B. Deformability of oxygenated irreversibly sickled cells. J Clin Invest. 65 (1), 189-196 (1980).
- Rabai, M., et al. Deformability analysis of sickle blood using ektacytometry. Biorheology. 51 (2-3), 159-170 (2014).
- Bessis, M., Mohandas, N. Laser Diffraction Patterns of Sickle Cells in Fluid Shear Fields. Blood Cells. 3, 229-239 (1977).
- Kim, Y., Kim, K., Park, Y. Blood Cell - An Overview of Studies in Hematology. Moschandreou, T. E. , InTech. (2012).
- Streekstra, G. J., Dobbe, J. G., Hoekstra, A. G. Quantification of the fraction poorly deformable red blood cells using ektacytometry. Opt Express. 18 (13), 14173-14182 (2010).
- Renoux, C., et al. Importance of methodological standardization for the ektacytometric measures of red blood cell deformability in sickle cell anemia. Clin Hemorheol Microcirc. 62 (2), 173-179 (2016).
- Parrow, N. L., et al. Measurements of red cell deformability and hydration reflect HbF and HbA2 in blood from patients with sickle cell anemia. Blood Cells Mol Dis. 65, 41-50 (2017).
- Ballas, S. K., Smith, E. D. Red blood cell changes during the evolution of the sickle cell painful crisis. Blood. 79 (8), 2154-2163 (1992).
- Johnson, R. M., Ravindranath, Y. Osmotic scan ektacytometry in clinical diagnosis. J Pediatr Hematol Oncol. 18 (2), 122-129 (1996).
- Mohandas, N., Clark, M. R., Jacobs, M. S., Shohet, S. B. Analysis of factors regulating erythrocyte deformability. J Clin Invest. 66 (3), 563-573 (1980).
- Lazarova, E., Gulbis, B., Oirschot, B. V., van Wijk, R. Next-generation osmotic gradient ektacytometry for the diagnosis of hereditary spherocytosis: interlaboratory method validation and experience. Clin Chem Lab Med. 55 (3), 394-402 (2017).
- Anderson, C., Aronson, I., Jacobs, P. Erythrocyte Deformability is Reduced and Fragility increased by Iron Deficiency. Hematology. 4 (5), 457-460 (1999).
- Reinhart, W. H., et al. Washing stored red blood cells in an albumin solution improves their morphologic and hemorheologic properties. Transfusion. 55 (8), 1872-1881 (2015).
- Shin, S., et al. Progressive impairment of erythrocyte deformability as indicator of microangiopathy in type 2 diabetes mellitus. Clin Hemorheol Microcirc. 36 (3), 253-261 (2007).
- Tu, H., et al. Low Red Blood Cell Vitamin C Concentrations Induce Red Blood Cell Fragility: A Link to Diabetes Via Glucose, Glucose Transporters, and Dehydroascorbic Acid. EBioMedicine. 2 (11), 1735-1750 (2015).
- Tiburcio, M., et al. A switch in infected erythrocyte deformability at the maturation and blood circulation of Plasmodium falciparum transmission stages. Blood. 119 (24), e172-e180 (2012).
- Henon, S., Lenormand, G., Richert, A., Gallet, F. A new determination of the shear modulus of the human erythrocyte membrane using optical tweezers. Biophys J. 76 (2), 1145-1151 (1999).
- Mills, J. P., Qie, L., Dao, M., Lim, C. T., Suresh, S. Nonlinear elastic and viscoelastic deformation of the human red blood cell with optical tweezers. Mech Chem Biosyst. 1 (3), 169-180 (2004).
- Moura, D. S., et al. Automatic real time evaluation of red blood cell elasticity by optical tweezers. Rev Sci Instrum. 86 (5), 053702(2015).
- Evans, E. A. New membrane concept applied to the analysis of fluid shear- and micropipette-deformed red blood cells. Biophys J. 13 (9), 941-954 (1973).
- Chen, X., Feng, L., Jin, H., Feng, S., Yu, Y. Quantification of the erythrocyte deformability using atomic force microscopy: correlation study of the erythrocyte deformability with atomic force microscopy and hemorheology. Clin Hemorheol Microcirc. 43 (3), 243-251 (2009).
- Musielak, M. Red blood cell-deformability measurement: review of techniques. Clin Hemorheol Microcirc. 42 (1), 47-64 (2009).
- Dobbe, J. G., Streekstra, G. J., Hardeman, M. R., Ince, C., Grimbergen, C. A. Measurement of the distribution of red blood cell deformability using an automated rheoscope. Cytometry. 50 (6), 313-325 (2002).
- Dobbe, J. G., et al. Analyzing red blood cell-deformability distributions. Blood Cells Mol Dis. 28 (3), 373-384 (2002).
- Kikuchi, Y., Arai, T., Koyama, T. Improved filtration method for red cell deformability measurement. Med Biol Eng Comput. 21 (3), 270-276 (1983).
- Moessmer, G., Meiselman, H. J. A new micropore filtration approach to the analysis of white cell rheology. Biorheology. 27 (6), 829-848 (1990).
- Guo, Q., et al. Microfluidic analysis of red blood cell deformability. J Biomech. 47 (8), 1767-1776 (2014).
- Doh, I., Lee, W. C., Cho, Y. H., Pisano, A. P., Kuypers, F. A. Deformation measurement of individual cells in large populations using a single-cell microchamber array chip. Appl Phys Lett. 100 (17), 173702-173703 (2012).
- Baskurt, O. K., et al. Comparison of three commercially available ektacytometers with different shearing geometries. Biorheology. 46 (3), 251-264 (2009).
- Baskurt, O. K., et al. New guidelines for hemorheological laboratory techniques. Clin Hemorheol Microcirc. 42 (2), 75-97 (2009).
- Uyuklu, M., et al. Effects of storage duration and temperature of human blood on red cell deformability and aggregation. Clin Hemorheol Microcirc. 41 (4), 269-278 (2009).
- Uyuklu, M., Meiselman, H. J., Baskurt, O. K. Effect of hemoglobin oxygenation level on red blood cell deformability and aggregation parameters. Clin Hemorheol Microcirc. 41 (3), 179-188 (2009).
- Embury, S. H., Clark, M. R., Monroy, G., Mohandas, N. Concurrent sickle cell anemia and alpha-thalassemia. Effect on pathological properties of sickle erythrocytes. J Clin Invest. 73 (1), 116-123 (1984).
- von Tempelhoff, G. F., et al. Correlation between blood rheological properties and red blood cell indices(MCH, MCV, MCHC) in healthy women. Clin Hemorheol Microcirc. 62 (1), 45-54 (2016).
- Da Costa, L., Galimand, J., Fenneteau, O., Mohandas, N. Hereditary spherocytosis, elliptocytosis, and other red cell membrane disorders. Blood Rev. 27 (4), 167-178 (2013).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved