A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
An Available Technique for Preparation of New Cast MnCuNiFeZnAl Alloy with Superior Damping Capacity and High Service Temperature
In This Article
Summary
Here we present a protocol to obtain a novel Mn-Cu-based alloy with excellent comprehensive performances by a high-quality smelting technology and reasonable heat treatment methods.
Abstract
Manganese (Mn)-copper (Cu)-based alloys have been found to have damping capacity and can be used to reduce harmful vibrations and noise effectively. M2052 (Mn-20Cu-5Ni-2Fe, at%) is an important branch of Mn-Cu-based alloys, which possesses both excellent damping capacity and processability. In recent decades, lots of studies have been carried out on the performance optimization of M2052, improving the damping capacity, mechanical properties, corrosion resistance, and service temperature, etc. The major methods of performance optimization are alloying, heat treatment, pretreatment, and different ways of molding etc., among which alloying, as well as adopting a reasonable heat treatment, is the simplest and most effective method to obtain perfect and comprehensive performance. To obtain the M2052 alloy with excellent performance for casting molding, we propose to add Zn and Al to the MnCuNiFe alloy matrix and use a variety of heat treatment methods for a comparison in the microstructure, damping capacity, and service temperature. Thus, a new type of cast-aged Mn-22.68Cu-1.89Ni-1.99Fe-1.70Zn-6.16Al (at.%) alloy with superior damping capacity and high service temperature is obtained by an optimized heat treatment method. Compared with the forging technique, cast molding is simpler and more efficient, and the damping capacity of this as-cast alloy is excellent. Therefore, there is a suitable reason to think that it is a good choice for engineering applications.
Introduction
Since the Mn-Cu alloys were found by Zener to have damping capacity1, they have received widespread attention and research2. The advantages of Mn-Cu alloy are that it has high damping capacity, especially at low strain amplitudes, and its damping capacity cannot be disturbed by a magnetic field, which is quite different from ferromagnetic damping alloys. The high damping capacity of Mn-Cu-based alloys can be mainly attributed to the movability of the internal boundaries, mainly including twin boundaries and phase boundaries, which are generated in the face-centered-cubic-to-face-centered-tetragonal (f.c.c.-f.c.t.) phase transition under the martensite transformation temperature (Tt)3. It has been found that Tt depends directly on the Mn content in the Mn-Cu-based alloy4,5; that is, the higher the Mn content, the higher the Tt and the better the damping capacity of the material. The alloy, which contains more than 80 at% manganese, was found to have high damping capacity and optimum strength when quenched from the solid-solution temperature6. However, the higher Mn concentration in the alloy would directly cause the alloy to be more brittle and have a lower elongation, impact toughness, and a worse corrosion resistance, which means the alloy will not meet the engineering requirements. Previous research findings revealed that an aging treatment under suitable conditions is an effective way to reconcile this problem; for instance, Mn-Cu-based damping alloys containing 50 - 80 at% Mn can also obtain a high Tt and favorable damping capacity by an aging treatment in the appropriate temperature range7. This is due to the decomposition of the γ-parent phase into nanoscale Mn-rich regions and nanoscale Cu-rich regions while aging in the temperature range of the miscibility gap8,9,10, which is considered to improve Tt of this alloy along with its damping capacity. Clearly, it is an effectual method which can combine high damping capacity with excellent workability.
M2052 alloy used for forging forming, a representative Mn-Cu-based high-damping alloy with medium Mn content developed by Kawahara et al.11, has been extensively studied in the last few decades. Researchers found that M2052 alloy has a good sweet spot between damping capacity, yield strength, and workability. Compared with the forging technique, casting has been widely used so far due to the simple molding process, low production costs, and high productivity, etc. The influential factors (e.g., oscillation frequency, strain amplitude, cooling velocity, heat treatment temperature/time, etc.) on the damping capacity, microstructure, and damping mechanism of M2052 alloy have been studied by some researchers12,13,14,15,16,17,18. Nevertheless, the casting performance of M2052 alloy is inferior, for instance, a wide range of crystallization temperature, the occurrence of casting porosity, and concentrated shrinkage, eventually resulting in the unsatisfactory mechanical properties of the castings.
The purpose of this paper is to provide the industrial field with a feasible method of obtaining a cast Mn-Cu based alloy with excellent properties which can be used in machinery and in the precision instruments industry to reduce vibration and ensure the product quality. According to the effect of alloying elements on the phase transformation and the casting performance, Al element is considered to reduce the γ-phase region and the stability of the γ phase, which can make the γ phase more easily transform into a γ' phase with micro-twins. Moreover, the solution of Al atoms in the γ phase will increase the strength of the alloy, which can improve the mechanical properties. Also, Al element is one of the important elements which can improve the casting properties of Mn-Cu alloy. Zn element is beneficial to improving the casting and damping properties of the alloy. Finally, 2 wt% Zn and 3 wt% Al were added to the MnCuNiFe quaternary alloy in this work and a new cast Mn-26Cu-12Ni-2Fe-2Zn-3Al (wt%) alloy was developed. Furthermore, several different heat treatment methods are used in this work and their distinct effects are discussed as follows. The homogenization treatment was used to reduce dendrite segregation. The solution treatment was used for impurities immobilization. The aging treatment is used for triggering spinodal decomposition; meanwhile, the various aging times are used for seeking out the optimizing parameters for both excellent damping capacity and a high service temperature. Ultimately, a preferable heat treatment method was screened for superior damping capacity, as well as a high service temperature.
It turns out that the maximum internal friction (Q-1) and the highest service temperature can be achieved concurrently by aging the alloy at 435 °C for 2 h. Because of the simplicity and efficiency of this preparation method, a novel as-cast Mn-Cu-based damping alloy with excellent performance can be produced, which is of important practical significance for its engineering application. This method is particularly suitable for the preparation of casting Mn-Cu-based high damping alloy which can be used for vibration reduction.
Protocol
1. Preparation of the Raw Materials
- Weigh all the required raw materials with an electronic scale by mass percentage (65% electrolytic Mn, 26% electrolytic Cu, 2% industrial pure Fe, 2% electrolytic Ni, 3% electrolytic Al, and 2% electrolytic Zn), as shown in Figure 1.
NOTE: All these raw materials were commercially available.

Figure 1: Presentation of raw materials. The materials used include 65 wt% electrolytic Mn, 26 wt% electrolytic Cu, 2 wt% industrial pure Fe, 2 wt% electrolytic Ni, 2 wt% electrolytic Zn, and 3 wt% electrolytic Al. Please click here to view a larger version of this figure.
2. Melting and Casting Process
NOTE: The detailed steps of sand casting are shown in Figure 2.
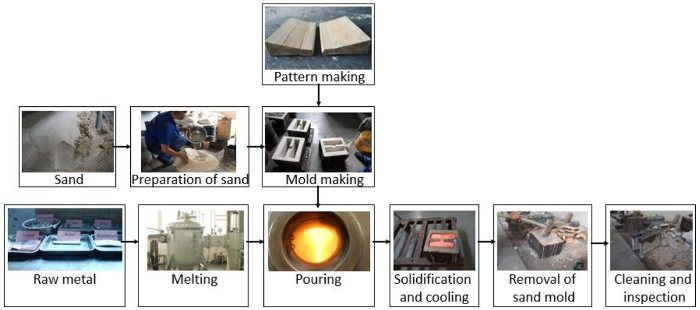
Figure 2: Sand casting and molding steps. The main process includes pattern-making, mold-making, and a casting operation. Please click here to view a larger version of this figure.
- To prepare patterns, make patterns according to the product drawing, and make sure that the size of the pattern is expanded to a certain extent to be liable for shrinkage and machining allowances.
NOTE: The pattern material used in this work is wood ( Figure 3) because a wood pattern is light, easy to work, and has a low cost and short production cycle.

Figure 3: Patterns used in the casting mold. These wood patterns were used to obtain the shape of the castings. Please click here to view a larger version of this figure.
- To prepare the molding sand, mix together the quartz sand with 4% - 8% sodium silicate.
NOTE: The sand diameter is about 0.4 mm and the particles are uniform. - Complete the main molding process by hands.
- First, put two patterns in the molding flask.
- Then, roll over the flask after ramming the molding sand around the patterns and withdraw the patterns from the sand.
- Finally, brush the surface of the sand mold with casting coating for improving the casting surface quality and reducing casting defects.
NOTE: The molded sand mold is shown in Figure 4. - To obtain a dry sand mold, put the mold in an oven at 180 °C and bake it for more than 8 h before casting to enhance its strength and permeability, facilitate the melt filling, and ensure the quality of the casting products.

Figure 4: The molded sand mold. It has two cavities and its surface has been covered with a coating. Please click here to view a larger version of this figure.
3. Induction of Melting
NOTE: Use a medium-frequency vacuum induction melting furnace.
- Open the furnace lid, put 20.8 kg of Mn, 8.32 kg of Cu, 0.64 kg of Ni, 0.64 kg of Fe, 0.64 kg of Zn, and 0.96 kg of Al materials in the crucible successively, and cover the materials with cryolite at last.
- Take out the casting mold from the oven and put it in the furnace; adjust its position for a successful pouring. Close the lid, vacuum the furnace, and then open the heat distribution system to start melting the alloy.
- When the metals start to melt, fill the furnace with argon to a 93-KPa negative pressure, to inhibit the splashing of the molten metal.
- After the alloy has melted, refine it for several minutes to reduce the harmful impurities and gas content.
NOTE: The melting procedure often includes smelting and refining.
4. Casting the Alloy
- Pour the molten metal smoothly into the casting mold after the refining process.
- After the molten metal is completely solidified, break the vacuum and take out the casting mold.
- Remove the castings from the casting mold when the temperature of the mold drops to a low level.
5. Pretreatment of the Castings
NOTE: The macrophotograph of the molded part is shown in Figure 5.
- Cut specimens from the casting by using a linear cutting machine.
NOTE: The specimens for the X-ray diffractometer (XRD) measurements and the metallographic observation are in 10 x 10 x 1 mm3. The specimens for the dynamic thermomechanical analysis (DMA) possess a dimension of 0.8 x 10 x 35 mm3.
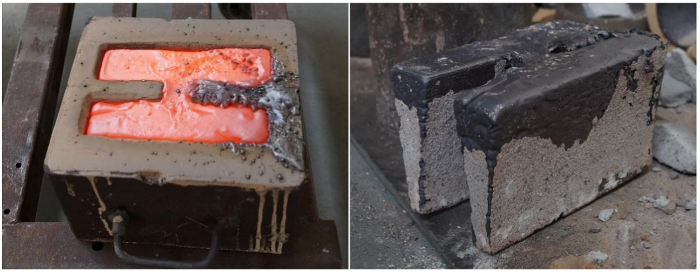
Figure 5: The molded parts in the sand mold and the removed parts. Two castings were molded at one time. Please click here to view a larger version of this figure.
6. Heat Treatment
- Divide the polished specimens into seven groups and keep specimen #1 free of treatment, maintaining an as-cast state for comparison. Put the others in a box-type resistance oven for different heat treatments.
- Homogenize specimens #2 and #5 at 850 °C for 24 h and, subsequently, quench them in cool water before aging them at 435 °C, specimen #2 for 4 h and specimen #5 for 2 h.
- Solution-treat specimens #3 and #6 at 900 °C for 1 h and, subsequently, quench them in cool water before aging them at 435 °C, specimen #3 for 4 h and specimen #6 for 2 h.
- Age specimens #4 and #7 at 435 °C for 4 h and 2 h, respectively.
7. Damping Capacity Test
- Use a Dynamic Mechanical Analysis (DMA) to measure the damping capacity of the specimens17.
NOTE: The test mode is strain sweep at room temperature. - During the test, detect the phase angle δ between the stress and the strain (as shown in Figure 6).
- Characterize the damping capacity by Q-1, which can be determined by the following formula.
Q-1 = Tan δ

Figure 6: The fixture construction and testing principle of the DMA. ( a) This panel shows the double cantilever fixture of the DMA. ( b) This panel shows the relationship of the applied sinusoidal stress to the strain and the resultant phase lag. The values of the lag between the stress and the strain, as well as the modulus, can be calculated by formulae. Please click here to view a larger version of this figure.
8. Sample Characterization
- Electrolytic polishing and metallographic observation
- For a dendrite microstructure observation, etch all specimens for about 1 min in a mixed solution of perchloric acid and absolute alcohol at 1:27.
- Then, clean the specimens with acetone, dry the sample with a blower, and observe the dendritic structure with a metallographic microscope.
- Phase structure characterization
- Characterize the phase structure and the lattice parameters of the specimens by X-ray diffraction (XRD) with CuKα radiation12,22.
NOTE: Use a scan speed at 2°/min. Before the XRD measurement, prepare the specimens carefully by removing any surface stress.
- Characterize the phase structure and the lattice parameters of the specimens by X-ray diffraction (XRD) with CuKα radiation12,22.
Results
Figure 7 shows the dependency of the damping capacity on the strain amplitude for the as-cast MnCuNiFeZnAl alloy specimens #1 - #7 and as-cast M2052. The results show that the damping capacity of specimen #1 is higher than that of cast M2052 alloy (as shown in Figure 7a) and the traditional forged M2052 high-damping alloy mentioned in previous articles20,21. Moreover...
Discussion
To ensure that this kind of as-cast Mn-Cu-based alloy possesses both superior damping capacity and excellent mechanical properties, it is necessary to ensure that the castings have a stable chemical composition, a high purity, and an excellent crystal structure. Therefore, strict quality control is necessary for the smelting, pouring, and heat treatment processes.
Firstly, it is necessary to choose the proper ingredients for the alloy. It should be considered that the added alloy elements...
Disclosures
The authors have nothing to disclose.
Acknowledgements
We give thanks to the financial support of the National Natural Science Foundation of China (11076109), the Hong Kong Scholars Program (XJ2014045, G-YZ67), the "1000 Talents Plan" of the Sichuan Province, the Talent Introduction Program of Sichuan University (YJ201410), and the Innovation and Creative Experiment Program of Sichuan University (20171060, 20170133).
Materials
| Name | Company | Catalog Number | Comments |
| manganese | Daye Nonferrous Metals Group Holdings Co., Ltd. | DJMnB | produced by electrolysis |
| copper | Daye Nonferrous Metals Group Holdings Co., Ltd. | Cu-CATH-2 | produced by electrolysis |
| Nickel | Daye Nonferrous Metals Group Holdings Co., Ltd. | Ni99.99 | produced by electrolysis |
| Iron | Ningbo Jiasheng Metal Materials Co., Ltd. | YT01 | industrial pure Fe |
| Zinc | Daye Nonferrous Metals Group Holdings Co., Ltd. | 0# | produced by electrolysis |
| Aluminum | Daye Nonferrous Metals Group Holdings Co., Ltd. | Al99.90 | produced by electrolysis |
References
- Zener, C. . Elasticity and anelasticity of metals. , (1948).
- Jensen, J. W., Walsh, D. F. Manganese-Copper damping alloys. Bulletin 624. , (1965).
- Wang, X. Y., Peng, W. Y., Zhang, J. H. Martensitic twins and antiferromagnetic domains in gamma-MnFe(Cu) alloy. Materials Science and Engineering A. 438, 194-197 (2006).
- Wang, X. Y., Zhang, J. H. Structure of twin boundaries in Mn-based shape memory alloy: a HRTEM study and the strain energy driving force. Acta Materialia. 55 (15), 5169-5176 (2007).
- Yin, F. X., Ohsawa, Y., Sato, A., Kawahara, K. Decomposition behavior of the gamma(Mn) solid solution in a Mn-20Cu-8Ni-2Fe (at%) alloy studied by a magnetic measurement. Materials Transactions,JIM. 40 (5), 451-454 (1999).
- Dean, R. S., Potter, E. V., Long, J. R. Properties of transitional structures in Copper-Manganese alloys. Metallurgical and Materials Transactions, ASM. 34, 465-500 (1945).
- Yin, F. X., Ohsawa, Y., Sato, A., Kawahara, K. Temperature dependent damping behavior in a Mn-18Cu-6Ni-2Fe alloy continuously cooled in different rates from the solid solution temperature. Scripta Materialia. 38 (9), 1314-1346 (1998).
- Findik, F. Improvements in spinodal alloys from past to present. Materials and Design. 42 (42), 131-146 (2012).
- Yan, J. Z., Li, N., Fu, X., Zhang, Y. The strengthening effect of spinodal decomposition and twinning structure in MnCu-based alloy. Materials Science and Engineering A. 618, 205-209 (2014).
- Soriano-Vargas, O., Avila-Davila, E. O., Lopez-Hirata, V. M., Cayetano-Castro, N., Gonzalez-Velazquez, J. L. Effect of spinodal decomposition on the mechanical behavior of Fe-Cr alloys. Materials Science and Engineering A. 527 (12), 2910-2914 (2010).
- Yin, F. X. Damping behavior characterization of the M2052 alloy aimed for practical application. Acta Metallurgica Sinica. 39 (11), 1139-1144 (2003).
- Yin, F. X., Ohsawa, Y., Sato, A., Kohji, K. Decomposition of high temperature gamma(Mn) phase during continuous cooling and resultant damping behavior in Mn74.8Cu19.2Ni4.0Fe2.0 and Mn72.4Cu20.0Ni5.6Fe2.0 alloys. Materials Transactions, JIM. 39 (8), 841-848 (1998).
- Sakaguchi, T., Yin, F. X. Holding temperature dependent variation of damping capacity in a MnCuNiFe damping alloy. Scripta Materialia. 54 (2), 241-246 (2006).
- Tanji, T., et al. Measurement of damping performance of M2052 alloy at cryogenic temperatures. Journal of Alloys and Compounds. 355 (1-2), 207-210 (2003).
- Yin, F. X., Iwasaki, S., Sakaguchi, T., Nagai, K. Susceptibility of damping behavior to the solidification condition in the as-cast M2052 high-damping alloy. Key Engineering Materials. 319, 67-72 (2006).
- Yin, F. X., Ohsawa, Y., Sato, A., Kawahara, K. Characterization of the strain-amplitude and frequency dependent damping capacity in the M2052 alloy. Materials Transactions, JIM. 42 (3), 385-388 (2001).
- Zhong, Z. Y., et al. Mn segregation dependence of damping capacity of as-cast M2052 alloy. Materials Science and Engineering A. 660, 97-101 (2016).
- Liu, W. B., et al. Novel cast-aged MnCuNiFeZnAl alloy with good damping capacity and high service temperature toward engineering application. Materials Design. 106, 45-50 (2016).
- Cowlam, N., Shamah, A. M. A diffraction study of y-Mn-Cu alloys. Journal of Physics F: Metal Physics. 11 (1), 27-43 (1981).
- Yan, J. Z., et al. Effect of pre-deformation and subsequent aging on the damping capacity of Mn-20 at.%Cu-5 at.%Ni-2 at.%Fe alloy. Advanced Engineering Materials. 17 (9), 1332-1337 (2015).
- Zhang, Y., Li, N., Yan, J. Z., Xie, J. W. Effect of the precipitated second phase during aging on the damping capacity degradation behavior of M2052 alloy. Advances in Materials Research. 873, 36-41 (2014).
- Yin, F. X., Ohsawa, Y., Sato, A., Kawahara, K. X-ray diffraction characterization of the decomposition behavior of gamma(Mn) phase in a Mn-30 at.% Cu alloy. Scripta Materialia. 40 (9), 993-998 (1999).
- Yin, F. X., Ohsawa, Y., Sato, A., Kawahara, K. Phase decomposition of the gamma phase in a Mn-30 at.% Cu alloy during aging. Acta Materialia. 48 (6), 1273-1282 (2000).
- Ritchie, I. G., Sprungmann, K. W., Sahoo, M. Internal-friction in Sonoston - a high damping Mn/Cu-based alloy for marine propeller applications. Journal De Physique. 46 (C-10), 409-412 (1985).
- Kawahara, K., Sakuma, N., Nishizaki, Y. Effect of Fourth Elements on Damping Capacity of Mn-20Cu-5Ni Alloy. Journal of the Japan Institute of Metals. 57 (9), 1097-1100 (1993).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved