Method Article
Automated Behavioral Analysis of Large C. elegans Populations Using a Wide Field-of-view Tracking Platform
In This Article
Summary
We describe protocols for using the wide field-of-view nematode tracking platform (WF-NTP), which enables high-throughput phenotypic characterization of large populations of Caenorhabditis elegans. These protocols can be used to characterize subtle behavioral changes in mutant strains or in response to pharmacological treatment in a highly scalable fashion.
Abstract
Caenorhabditis elegans is a well-established animal model in biomedical research, widely employed in functional genomics and ageing studies. To assess the health and fitness of the animals under study, one typically relies on motility readouts, such as the measurement of the number of body bends or the speed of movement. These measurements usually involve manual counting, making it challenging to obtain good statistical significance, as time and labor constraints often limit the number of animals in each experiment to 25 or less. Since high statistical power is necessary to obtain reproducible results and limit false positive and negative results when weak phenotypic effects are investigated, efforts have recently been made to develop automated protocols focused on increasing the sensitivity of motility detection and multi-parametric behavioral profiling. In order to extend the limit of detection to the level needed to capture the small phenotypic changes that are often crucial in genetic studies and drug discovery, we describe here a technological development that enables the study of up to 5,000 individual animals simultaneously, increasing the statistical power of the measurements by about 1,000-fold compared to manual assays and about 100-fold compared to other available automated methods.
Introduction
Approximately half a century ago, Sydney Brenner introduced Caenorhabditis elegans (C. elegans) as a model system to study development and neurobiology, as this small (1 mm in length), transparent nematode worm is easy to manipulate genetically and to maintain in the laboratory1. Today, C. elegans is used to study a wide variety of biological processes including apoptosis, cell signaling, the nature of the cell cycle, gene regulation, metabolism, and ageing2. Furthermore, the cellular and tissue complexity, protein expression patterns and the conservation of disease pathways between C. elegans and higher organisms (80% of worm genes have a human orthologue), linked with the simplicity and cost-effectiveness of cultivation, make it an effective in vivo model organism amenable to high-throughput genetic3,4,5,6,7,8,9,10,11,12,13 and drug14,15,16 screenings. For all these reasons, C. elegans has been employed for the characterization of normal and disease-related molecular pathways; in the field of neurodegeneration, for example, it has enabled the exploration of the effects of ageing on protein aggregation3,4,7,15,17,18, and the characterization of promoters and inhibitors of protein aggregation3,4,5,6,7,14,18.
The overall fitness of the worms, which is an important behavioral parameter to be defined in this type of study, can be measured manually in a variety of ways, such as by counting the number of body bends per minute (BPM)4,6,19, or by measuring the speed of movement20,21,22, as well as by measuring the average lifespan and rates of paralysis. Although manual measurements of body bends and speed of movement have led to many important insights into a variety of molecular pathways and mechanisms3,4,14,19,20,23, manual assays remain currently low-throughput, highly labor-intensive, and time-consuming whilst being prone to errors and to human biases. These issues present considerable challenges in the collection of data with sufficient statistical power to distinguish subtle behavioral changes. This limitation is particularly relevant for drug screening as treatments with potential drug molecules often lead to small phenotypic changes24, therefore requiring the study of large numbers of animals in order to acquire reproducible results. To illustrate this point, recent studies have shown that a high power of detection (POD) is necessary to define with confidence any significant changes in behavior and to limit false positive results25. This has resulted in a strong motivation in the C. elegans community to replace manual counting with reproducible, automated, time- and cost-effective measurements. To meet this demand, several laboratories have recently developed methods for high-sensitivity measurements and accurate worm tracking of larger number of worms22,26,27,28,29,30,31,32,33.
In order to expand further the process of automation to the large cohorts of animals needed for statistically significant measurements, we have recently developed a wide field-of-view nematode tracking platform (WF-NTP)15,34,35,36, which enables the simultaneous investigation of multiple phenotypic readouts on very large worm populations, a key factor in statistically relevant phenotypical detection25. Not only can the WF-NTP currently monitor up to 5,000 animals in parallel, but the phenotypic readouts also include multiple parameters, including the rate and amplitude of body bends, the speed of movement, the fraction of the population that is paralyzed, and the size of the animals. It is therefore readily possible to screen thousands of worms in parallel and to combine the different readouts into a behavioral map to create a multidimensional fingerprint36. The associated open-source software is written in Python, which is also required to operate it and is completely customizable. A graphical user interface (GUI) is also provided to enable users to adopt this technology.
Here, we present a series of protocols that illustrate some of the potential applications of the WF-NTP. In particular, we discuss the administration of compounds, ranging from small molecules to protein therapeutics, and describe how to screen their effects directly over large populations of worms, thus effectively removing the need to sample small sub-populations. The use of the WF-NTP for such a purpose has already brought significant advantages in procedures aimed to design drug discovery programs of Alzheimer's disease (AD)15,34,35 and Parkinson's disease (PD)18 using in vivo data for the assessment of therapeutic candidates35,37.
Protocol
1. Preparation of Materials for C. elegans Protocols
- To prepare a 10x M9 buffer solution, add 30 g of monobasic potassium phosphate, 60 g of dibasic sodium phosphate, and 50 g of sodium chloride to a 1 L autoclavable bottle.
- Add 1 L of purified water and autoclave at ca. 121 °C for 15 min.
- Dilute 10 times in purified water and autoclave at ca. 121 °C for 15 min.
- When cooled, add 1 mL of a solution of 1 M magnesium sulphate.
NOTE: Only 1x M9 (M9) should be used for worm handling in the following steps.
- To prepare the nematode growth medium (Rich-NGM), mix 2.7 g of sodium chloride, 15.75 g of agar, 5.75 g of casein, 900 mL of purified water, and autoclave at 121 °C for 15 min.
- Transfer the Rich-NGM medium to a warming oven (ca. 70 °C) if not used immediately; the Rich-NGM may be kept molten at ca. 70 °C for up to 12 h before becoming unusable.
- To the Rich-NGM mix, add 900 µL of a solution of 1 M calcium chloride, 900 µL of a solution of 1 M magnesium sulphate, and 900 µL of a solution of 5 mg/mL cholesterol in absolute ethanol.
NOTE: Cholesterol does not require autoclaving prior to use. - To prepare the experimental plates that maintain an age synchronous population, add 92 µL of 5-fluoro-2’-deoxyuridine (FUDR) at 812 µM to 900 mL of Rich-NGM.
NOTE: FUDR is both toxic and carcinogenic, so it is essential to wear nitrile gloves in addition to lab coats and safety glasses. Do not allow waste to enter general waste stream and dispose of by incineration with an afterburner. - Pour 20 mL of the autoclaved Rich-NGM into a 9 cm sterile Petri dish to generate a growth plate (Rich-NGM plate). Pour 15 mL of the autoclaved Rich-NGM into a 9 cm sterile Petri dish to make a motility plate (Mot plate). Pour 20 mL of Rich-NGM with FUDR into a 9 cm sterile Petri dish to generate an FUDR plate.
NOTE: The low agar volume of the Mot plate facilitates the camera focus and worm tracking (see step 3.2.5).
- Prepare the OP50 Escherichia coli strain, by autoclaving 1 L of LB broth at 121 °C for 15 min and inoculate with starter culture when cooled.
- Allow the bacterial culture to grow overnight at 37 °C and sterilize the tube to be used for centrifugation.
- Transfer the bacteria to sterile tubes and centrifuge at 4,600 x g for 15 min.
- Decant the supernatant and, using sterile water, suspend in 10% of the original volume to create 10x (10 times concentrated) OP50 stock.
- Dilute 15 mL of the 10x OP50 stock to 150 mL to generate 1x OP50, using sterile water, before being used to seed Rich-NGM plates. Use the remaining 10x solution to seed FUDR plates.
NOTE: The Mot plates will remain unseeded and will be used only in the image acquisition step.
- To seed plates with bacteria, segregate the Rich-NGM and FUDR plates.
- Under sterile conditions, add 350 µL of 1x OP50 to Rich-NGM plates and spread evenly.
NOTE: Do not spread to the edge of the plate as worms will crawl up the sides of the plate and die. - Under sterile conditions, add 350 µL of 10x OP50 to FUDR plates and spread evenly.
- Allow the plates to dry and incubate at 20 °C for 2 d before use.
- Under sterile conditions, add 350 µL of 1x OP50 to Rich-NGM plates and spread evenly.
2. Preparation of C. elegans for Use with the WF-NTP
- Maintain C. elegans on Rich-NGM plates or by transferring a small amount of agar containing worms face down onto a fresh bacterial layer. Maintain the C. elegans strains in this manner prior to initiating the experimental protocols.
- Using the transferring technique (see step 2.1), seed 20 plates of each strain using well-fed, mixed stage worms and allow them to grow for 2 d at 20 °C.
- Wash off 5 plates containing worms using 15 mL of M9 and pool in one 15 mL tube. Repeat for all 20 plates of each strain.
- Centrifuge the worms at 2,000 x g for 2 min, remove the supernatant, and suspend in 2 mL of M9 solution.
- Prepare 10 mL of bleaching solution by mixing 13% sodium hypochlorite and 4 M sodium hydroxide at a ratio of 3:2 vol/vol.
NOTE: This solution does not require autoclaving. Both components and the resulting solution are corrosive and should be handled with care using nitrile gloves. Sodium hydroxide is mildly corrosive to glass and should be stored in plastic containers. - Add 1 mL of bleaching solution to each centrifuge tube and shake vigorously for approximately 3.5 min or until the cuticle has fully dissolved.
- Under sterile conditions, dilute the samples to 15 mL M9 and centrifuge at 2,000 x g for 2 min. Remove the supernatant and suspend in 15 mL of M9 solution.
- Repeat step 2.7 6 times, until only worm eggs remain, and the solution no longer has an odor of chlorine.
- Centrifuge the samples at 2,000 x g for 2 min, remove the supernatant, and suspend in 2 mL of M9 solution.
- Under sterile conditions, use a sterile glass pipette to transfer 2 mL of M9 containing eggs into each well of a 12-well tissue culture plate and incubate at 20 °C for a minimum of 16 h.
NOTE: Use separate plates for each strain to prevent cross contamination. The transferring step from the centrifuge tubes to the multi-wells helps to avoid worm suffocation. - Transfer the worms hatched overnight from the 12-well plates into 15 mL centrifuge tubes. Take 3 drops of 5 µL of worm solution and count the number of worms present at first larval stage (L1).
- Calculate the number of worms per µL of solution.
- Seed L1 worms onto Rich-NGM plates at 3,000 worms per plate and allow them to grow for 2 days at 20 °C, until they reach last larval stage (L4) stage.
3. General WF-NTP Protocol
- Add 2.2 mL of each drug compound at the appropriate concentration (such as 25 or 10 μM for drugs like squalamine18 and bexarotene15, respectively) to 6 FUDR plates and allow to dry under sterile conditions for each desired worm strain.
NOTE: This step is repeated for each compound under examination, for each concentration and each solvent used.- Wash the worms off 5 Rich-NGM plates prepared at step 2.13 using 15 mL of M9 buffer and transfer the worms to a centrifuge tube.
- Centrifuge at 2,000 x g for 2 min, remove supernatant, and suspend in 3 mL of M9. Take 3 drops of 5 µL of the M9 solution containing worms and count the number of worms present at the last larval stage L4.
- Calculate the number of worms per µL of M9 buffer.
- Seed 700 L4 larvae onto 6 of the dry FUDR plates containing a given compound, repeat for each concentration of each compound and allow to dry under sterile conditions.
- Incubate the worms at 24 °C from L4 for those strains where the worm paralysis is induced only by raising the temperature, such as the AD strain38. Count the day following the L4 larval stage as D0 of adulthood.
- Turn on the stage lights for the tracker.
- Clean the glass stage with 70% ethanol and ensure that no visible residue remains, then remove lens cap. Clean the imaging lens using an air duster. Ensure that the camera is plugged in correctly and installed and start recording with the image capture software.
- Adjust the camera settings to record 20 frames/s, with mono 16 recording parameters setup; record 1,200 frames, saving in MJPEG format at a 95% compression rate. Use an empty 9 cm Petri dish to ensure that the stage is set to the correct height and adjust it if necessary.
NOTE: Ensure that the edges of the plate are visible in the recording as this will allow the ‘keep-dead’ algorithm to work correctly (Figure 1a). - Under sterile conditions, take 2 plates previously prepared at step 3.2 with the same conditions and 1 Mot screening plate prepared in step 1.2.4. Add 3 mL of the M9 solution to the Mot plate. Use other 2 mL of M9 to wash 1/3 of the surface area of 2 plates prepared at step 3.2 onto the Mot plate.
- Place the Mot plate containing approximately 5 mL of the M9 solution and approximately 600 worms onto the tracker stage. Focus the camera using an individual worm and begin recording.
- When the recording is complete, discard the Mot plate with the worms; mark the FUDR plates as being used once and return it to the incubator.
- Repeat steps 3.3.3 to 3.3.5 for each experimental condition.
- Repeat steps 3.3.3 to 3.3.6 for each time point in the adult lifespan.
NOTE: The screening procedures can be carried out at any point of the worm’s adult lifespan (ca. 3 weeks). These protocols facilitate screening up to 9 time points; the authors recommend screening every 2 days starting from day 1 of adulthood.
4. Optimization for Discrete Dose Studies
- Prepare worms by repeating steps 3.1.1 to 3.1.3.
- Seed ca. 1000 L4 worms onto 5 FUDR plates. Incubate the plates at 24 °C until D3 of adulthood, for experiments involving AD38 worms.
- Using a sterile glass pipette, transfer the worms prepared under sterile conditions from the plate to a 15 mL centrifuge tube, centrifuge at 2,000 x g for 2 min and remove the supernatant.
- Suspend the solution of worms in 1 mL of M9 buffer. Transfer the worms to a microcentrifuge tube, centrifuge at 300 x g for 2 min, and remove the supernatant.
- For protein transduction, add 40 µL of the transfection delivery reagent and 20 µL of native protein at the desired concentration (typically 40 µM), and incubate at room temperature for 30 min.
- Under sterile conditions, use a sterile glass pipette to transfer worms from step 4.2.1 into a microcentrifuge tube containing encapsulated protein to give a total volume of 1060 µL. Place tubes horizontally and shake at 60 rpm in a 24 °C shaking incubator for 8 h.
- Under sterile conditions, add 4 mL of M9 solution to a Mot plate and transfer one microcentrifuge tube of worms plus drug onto the Mot plate.
- Set up the tracker by repeating steps 3.3 to 3.3.2.
- Under sterile conditions, add 4 mL of M9 solution to a Mot plate and transfer one microcentrifuge tube of worms plus drug onto the Mot plate.
- Place the Mot plate onto the tracker stage, focus the camera on an individual worm, and begin recording. When complete, label the Mot plate and set to one side.
- Repeat steps 4.4.1 and 4.4.3 for all samples.
- Using a glass pipette, transfer worms under sterile conditions from the Mot plate to a 15 mL centrifuge tube, and centrifuge at 2,000 x g for 2 min. Remove supernatant, transfer the pellet onto an FUDR plate, and allow to dry under sterile conditions.
- Repeat step 4.4.5 for all samples.
- Incubate the worms at 24 °C until D6 of adulthood.
NOTE: Multiple antibody incubations are also possible. - Add 3 mL of the M9 buffer to the Mot plate and use 2 mL of M9 solution to wash all the worms from an FUDR plate onto the Mot plate.
- Set up the tracker by repeating steps 3.3 to 3.3.2.
- Place the Mot plate onto the tracker stage, focus the camera as necessary, and begin recording. When the recording is complete, discard the Mot plate or recover it for further screening if desired.
- Repeat steps 4.6 to 4.6.2 for all samples.
5. Analyzing the Video Data
- After acquiring the videos, select the appropriate parameters in the software GUI (Figure 2) for data analysis.
- Load single or multiple videos via the Browsing function. Select an output destination folder. Ensure that 600 to 1,200 frames are used for a 30 s analysis.
NOTE: Any range of frames can be analyzed. - Insert a pixel to mm conversion factor, which will be 0.029 for imaging a 9 cm Petri dishes at full resolution. Select the tracking algorithm ‘keep dead’.
NOTE: The conversion factor will depend on the field of view used. Z-transform algorithm can be also used as an alternative. - Fill in the parameters in the Locating section (Figure 2): Z use images = 100, Z padding = 3, Std pixels = 54, Threshold = 9, Opening = 1, Closing = 2. Adjust the Filtering parameters to Minimum size = 20, Maximum size = 180, Worm-like = 0.94.
- Tune the Forming trajectories parameters: Maximum distance move = 10; Minimum length = 150, Memory = 10.
- Set Bends and Velocity parameters. Use 1.8 for Bend threshold, 0 for Minimum bends, and 150 for Frames to estimate velocity.
- Tune the Dead worm statistics. Use 5.0 for Maximum beat per minute and 1.0 for Maximum velocity.
- Choose the output folder. Set the number of Output frames to 50. Set Font size to 10.
NOTE: Optionally, select one or more Regions of interest (ROIs).
- Load single or multiple videos via the Browsing function. Select an output destination folder. Ensure that 600 to 1,200 frames are used for a 30 s analysis.
- Test the parameters by using the Example function. Output the example images and check that the worms are visible throughout the 8 thresholding steps (Figure 1).
- Use the Start job function.
- Analyze the text result files by combining the individual results for the whole dataset. Use the Export to tsv function in the GUI.
NOTE: Optionally, individual metrics of the worms can also be considered for population studies.- Analyze data with a statistical software package.
Results
The ease of operating, multiparametric analysis, and high throughput of the illustrated WF-NTP protocol (Figures 1 and 2) makes it possible to determine very small changes in worm behavior in a very accurate manner. The imaging platform is based on custom-made opto-mechanics, and it can be assembled using a 6 MP monochrome camera combined with 16 mm focal length high-resolution lens for 1'' sensor, illuminated with a 8'' by 8'' white backlight (see Table of Materials and also reference36 for additional details). The associated WF-NTP software is written in Python and was developed to run on Windows platforms. It runs on a custom assembled computer with 3.00 GHz octa-core processor and 64 GB of random-access memory (RAM). The software was also designed to parallelize the work and video analysis based on the RAM and CPU of the computer; i.e., a machine with a lower calculation power will result in less videos run in parallel. The setup we are currently using is optimized to run up to ca. 16 videos in parallel and can complete an analysis of ca. 100 videos overnight. Moreover, the high level of detail of customization provided in the GUI of the WF-NTP allows great control of the quality of the imaging analysis. The GUI can be used to directly upload large datasets in parallel or individual videos; specific frames can also be selected for detailed sub-analysis, together with a pixel conversion factor, which can be used to estimate behavioral metrics. One of the two different tracking algorithms (keep dead and Z-transform) can be chosen depending on whether or not the user would like to consider paralyzed animals in the analysis. The thresholding parameter can be tuned accordingly with the video and experimental quality. The opening and closing parameters allow the user to remove the noise and further implement the thresholding functionality. The skeletonizing algorithm offers an alternative method of analysis. The object size cut-offs (filtering) provide an additional filter for background noise and the worm-like parameter allows the user to consider worms only objects with an ellipsoidal shape, hence distinguishing from other objects that may have the same pixel size of the worms. After these thresholding operations, all the resulting labelled regions can be identified as individual worms and the positions of those regions are then stored for each frame for subsequent analysis and tracking. The eccentricity of each tracked worm is used to estimate the worm metrics such as the extent of worm bending (BPM) as a function of time. The users are also allowed to select the number of frames used to keep a worm in the memory of the software following collisions, and the number of pixels that a worm can move between frames can also be tuned to distinguish the animals from the noise. The minimum track length option allows the user to discard worms that have been tracked for only a few frames. Other key parameters, such as bends and velocity, allow the user to select the degree of bending necessary to be counted as a body bend and the number of frames considered to be needed to estimate the speed of the animals. Cut-off parameters can be further tuned for the inclusion of paralyzed animals. The output is automatically shown in the result files. These values are considered as upper limits for the evaluation of the fraction of paralyzed animals. The user can also select one or more regions of interest. This feature is particularly useful to analyze subpopulations of the worms and the output is sorted automatically in the results files. The output option allows the user to select the output folder and the number of tracking frames that will be produced for it. Various tool sets can also be used for further data analysis, such as the plot path tool that shows individual worm tracks and the fingerprinting tool that allows the user to create fingerprint maps.
This methodology enables new approaches to be adopted, not only for biological studies of C. elegans but also for pharmacological and medical research purposes, such as high-throughput screening of genetic modifications and drug treatments. We have illustrated this potential by describing the characterization of the phenotypes for large population studies (N > 1000) of various worm models of neurodegenerative disease (frontotemporal dementia (FTD)39, Parkinson's disease (PD)7, Alzheimer's disease (AD)16,40, and amyotrophic lateral sclerosis (ALS)19 (Figure 3a), and characterizing the effects of potential therapeutic molecules using worm models of PD18 and AD15,12 (Figure 3b). Two small molecules, squalamine18 and bexarotene15, were administered at concentrations up to 25 µM to PD (Figure 3b) and 10 µM to AD38 (Figure 3b) worms, respectively. Both compounds showed clear dose-dependent effects over the range of concentrations tested. We have shown that this high accuracy of the measurements is achieved by increasing the number of worms that can be analyzed compared to traditional methods (Figure 3c). We illustrated the importance of the sample size (Figure 3c) in molecular screening as well as in characterization of mutant strains. The increase in temperature from 20 °C leads to approximately half of the AD worms becoming paralyzed after 3 days of adulthood. Worm populations were screened under different conditions, e.g., when worms over-expressing the 42-residue form of the amyloid-β peptide (Aβ1-42) (AD worms) were exposed to subtle temperature variations (Figure 3c, left panel), when Aβ1-42 was expressed in all the neurons (Figure 3c, central panel), or when AD worms were exposed to bexarotene (Figure 3c, right panel). Worms were also analyzed from small ROIs randomly selected from the full field of view of the videos acquired with the WF-NTP (N < 50, yellow bars) highlighting the comparison of the motility of these worms with the average motility of the whole worm population (N < 1,000). In all the panels, the difference measured on the whole worm population appears to be statistically significant with p ≤ 0.0001 (****).
The WF-NTP protocol described here also allows the simultaneous recording of multiple parameters (Figure 1b) to support, in an optimal manner, both internal validation and the development of a comprehensive fingerprint of a wide range of conditions relative to a control sample, allowing for meaningful comparisons across multiple studies. This multi-parametric approach includes the simultaneous analysis of multiple behavioral features, including bending frequency, speed, paralysis rate, size, bend amplitude, and bend displacement36. This allows thousands of animals to be monitored in great detail and at a very high sensitivity and statistical significance and provides opportunities for large populations studies. This tracking procedure also has the advantage of allowing paralysis studies to be performed in parallel with other behavioral measurements, a key feature in molecular screening studies.
The results that have been achieved so far in AD15,34,35 and PD18 drug discovery demonstrate the importance of wide field-of-view data acquisition to greatly increase the numbers of animals that can be monitored in a single experiment, significantly decreasing the experimental errors and greatly improving the statistical validity of the studies. Based on these results, we anticipate that the WF-NTP protocol, which we made readily available for the community36, will significantly extend the use of C. elegans.
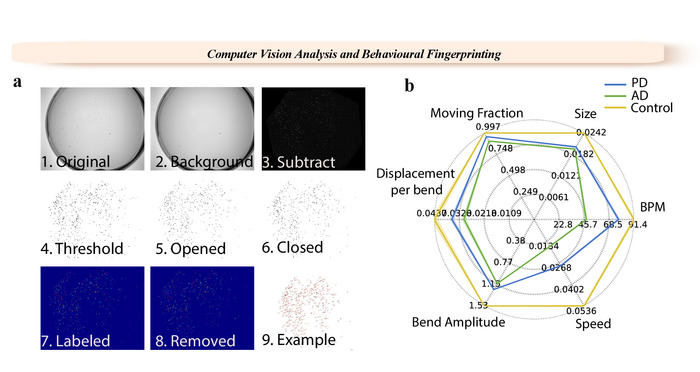
Figure 1: WF-NTP analysis steps and example of a fingerprint. (a) 1. Original video. 2. Background image. 3. Background subtracted image. 4-6. Thresholding steps. 7-9. Single labelling of worms.(b) Multiple phenotypes are reported with a fingerprint for wild-type worms and worm models of PD and AD. Please click here to view a larger version of this figure.
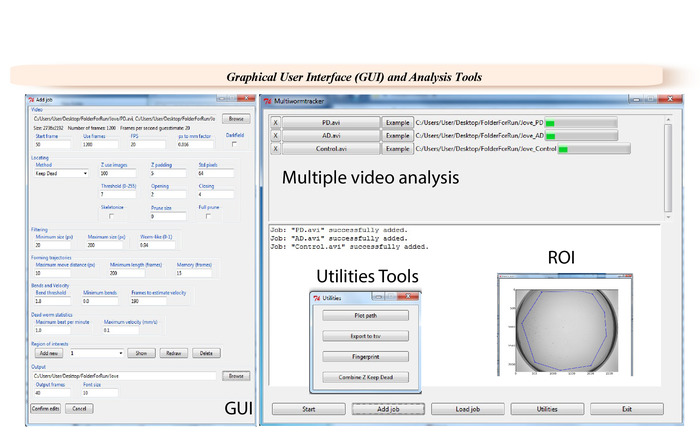
Figure 2: Graphical user interface (GUI) of the WF-NTP. Specific videos and selected frames can be selected in the GUI for analysis, and a pixel conversion factor can be inserted, after which the analysis is carried out with one of the two given tracking algorithms. It is possible to select the degree of bending necessary to count as a body bend as well as the number of frames needed to estimate the speed of the animals. Body bends and speed thresholds can determine the paralyzed worm statistics. Please click here to view a larger version of this figure.
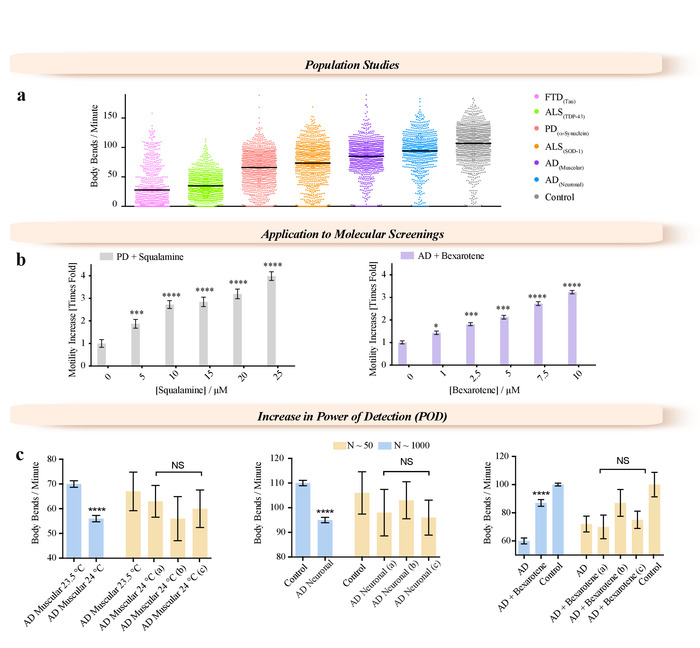
Figure 3: Examples of applications enabled by the WF-NTP method. (a) Application of WF-NTP in large population studies (N > 1,000) of BPM measurements for C. elegans models of a range of neurodegenerative diseases including FTD, PD, AD, and ALS. (b) Application of WF-NTP in drug discovery. (c) Importance of the population studies in temperature sensitivity, drug efficacy, and mutant strain characterization. Phenotypes of subpopulations N < 50 (yellow bars) are compared with those of the whole worm population (N < 1,000). The error bars indicate the standard error of the mean (SEM). Please click here to view a larger version of this figure.
Discussion
Because of the rapid expansion of techniques within the field of optical sciences, it is now possible to address the requirement for automated methods in C. elegans studies in substantially new ways. As a result, a number of digital tracking platforms20,41,42,43,44,45,46 have been designed and made available over the last few years in order to replace manual counting of parameters such as speed of movement, bending frequency, paralysis rate, as well as more complex forms of behaviors such as omega turns, and lifespan measurements. Most recent automated platforms have greatly improved the reproducibility and sensitivity of C. elegans studies41 and provided high quality data on small cohorts or even individual animals. We decided to extend the automation of the analysis of worm behavior to make it also possible to evaluate the phenotypes of cohorts of thousands of animals in parallel. The main advantage of the approach of studying worm cohorts is that it allows for accounting for the high intrinsic variability of worm behavior24 and for the fact that drug treatment studies often lead to subtle phenotypic variations, which are difficult to detect with sufficient statistical significance when using a small group of animals. A high power of detection (POD) is indeed necessary to detect with confidence any significant variation in behavior and to limit false positive results25.
Here, we have described a series of protocols based on a recently reported automated screening method for C. elegans, the wide field-of-view nematode tracking platform (WF-NTP)36. The protocol described here is divided into 5 parts. Parts 1 and 2 describe the preparation of large worm populations. Critical steps are the sterility of the working conditions and preparation of reagents and plates necessary to run the experiments. We note that, due to the increased throughput provided by this protocol compared to other screening methodologies36, it also requires increased quantities of reagents; this factor needs to be considered carefully in the experimental design. We also note that the bleaching step is critical and needs to be tested in advance as a large number of eggs and healthy larvae are necessary to run these experiments. Part 3 of this protocol details how to deliver drugs in solid media and screen worm populations. We note that this part of the protocol is strongly dependent on the number of drugs and drug concentrations to be tested by the user in parallel. The complete automation of the screening procedure and rapid data acquisition shift the limiting step from behavioral observation to reagent preparation and growth and synchronization of large worm populations. The key steps during the behavioral screening are the timings of the recording and any worm handling steps (e.g., the transfer of worms from the NGM plates to the tracking platform). The protocol described here is an example designed to screen the worms for up to 9 days during the adult lifespan; however, this protocol can be easily adapted to screen as many time points as the user desires, e.g., 18 consecutive days36. Part 4 then illustrates the application of the protocol to deliver protein molecules (e.g., antibodies and molecular chaperones) into C. elegans, and shows how the protocol illustrated in Parts 1-3 can be easily customized, depending on the desired application. We demonstrate how this procedure can be extended not only to the delivery of drug-like molecules but also for the administration of molecular chaperones or antibodies37. The first four steps (parts) are carried out under sterile conditions, unless noted otherwise. All liquid components should be autoclaved prior to use and the incubation steps should be performed at 70% relative humidity. In Part 5, we describe how to use the software package provided in combination with the tracking stage. This software has been custom designed for the analysis of WF-NTP data related to the behavior of large worm populations. We suggest that the user follows the guidelines provided in Part 5 for the data analysis; however, these parameters are dependent on the specific features of the recorded videos (i.e., fps, field of view, video resolution, number of acquired frames). The example function provided in the GUI has been designed to facilitate the evaluation of the correct parameters prior to the analysis.
These series of protocols make it possible to analyze the phenotypes of large populations of C. elegans (currently up to 5,000 individual worms in parallel) effectively, reducing artefacts due to the intrinsic variability of the behavior of the animals, in agreement with preliminary studies on the power of detection necessary to achieve statistical significance for studies of C. elegans25. The platform uses a system of high-resolution cameras, capable of recording images of large numbers of animals at a high speed, while simultaneously recording multiple large cohorts. The high performance and high throughput of the WF-NTP protocol makes it possible to determine very small changes in worm behavior in a very accurate manner. Therefore, this methodology enables new approaches to be considered not only for the study of the biology of C. elegans, but additionally for pharmacological and medical research, such as the high-throughput screening of genetic modifications and drug treatments. This procedure also has the advantage of allowing paralysis studies to be performed in parallel with other behavioral measurements, a key feature in molecular screening studies.
The results that have been achieved so far in the drug discovery programs of AD15,34,35 and PD18 demonstrate the importance of wide field-of-view data acquisition in substantially increasing the numbers of animals that can be monitored in a single experiment, thereby significantly decreasing the experimental errors and greatly improving the statistical validity of studies. While the current approach described in this protocol has focused on addressing challenges in the field of drug discovery, we hope that the methodology will be widely adopted in the community, and that its application will be extended to complex genetic and behavioral studies and expand the number of phenotypes that are currently detectable.
Disclosures
The authors declare that there are no conflicts of interest.
Acknowledgements
This work was supported by the Centre for Misfolding Diseases (CMD). FAA is supported by a Senior Research Fellowship award from the Alzheimer's Society, UK (Grant Number 317, AS-SF-16-003). The C. elegans strains were obtained from the Caenorhabditis elegans Genetic Centre (CGC).
Materials
| Name | Company | Catalog Number | Comments |
| Consumable reagents | |||
| monobasic potassium phosphate | Sigma Aldrich | P0662 | |
| dibasic sodium phosphate | Sigma Aldrich | S3264 | |
| sodium chloride | Sigma Aldrich | 13422 | |
| magnesium sulphate | Sigma Aldrich | M7506 | |
| Agar | Sigma Aldrich | A1296 | |
| Difco casein digest | Scientific Laboratory Supplies | 211610 | |
| calcium chloride dihydrate | Sigma Aldrich | C3881 | |
| cholesterol | Acros | 110190250 | |
| absolute ethanol | Vwr | 20821.33 | |
| 5-Fluoro-2'-deoxyuridine 98% | Alfa Aesar | L16497.ME | |
| LB medium capsules | MP biomedicals | 3002-031 | |
| 13% sodium hypochlorite | Acros Organics | AC219255000 | |
| Sodium hydroxide | Fisher Chemical | S/4880/53 | |
| Name | Company | Catalog Number | Comments |
| Strains | |||
| E. coli strain OP50 | Supplied by CGC | Op50 | E coli strain |
| C. elegans strain wild type | Supplied by CGC | N2 | C. elegans strain |
| C. elegans strain AD | Supplied by CGC | GMC101 | C. elegans strain |
| C. elegans strain PD | Supplied by CGC | NL5901 | C. elegans strain |
| C. elegans strain ALS | Supplied by CGC | AM725 | C. elegans strain |
| C. elegans strain Tau | Supplied by CGC | BR5485 | C. elegans strain |
| Name | Company | Catalog Number | Comments |
| Equipment | |||
| Tactrol 2 Autoclave | Priorclave | ||
| 9 cm sterile Petri dishes | Fisher Scientific | 11309283 | |
| 2 L erlenmeyer flasks | Scientific Laboratory Supplies | FLA4036 | |
| Nalgene 1 L Centrifuge pots | Fisher Scientific | 3120-1000 | |
| RC5C plus floor mounted centrifuge | Sorvall | 9900884 | |
| 15 mL centrifuge tubes | Fisher Scientific | 05-539-12 | |
| Heraeus Multifuge X3R | Thermofisher scientific | 75004515 | |
| Inoculating Spreaders | Fisher Scientific | 11821741 | |
| M4 multipette | Eppendorf | 4982000012 | |
| P1000 pipette | Eppendorf Research Plus | ||
| P200 pipette | Eppendorf Research Plus | 3123000055 | |
| P10 pipette | Eppendorf Research Plus | 3123000020 | |
| 1,000 μL low retention tips | Sarstedt | ||
| 300 μL low retention tips | Sarstedt | 70.765.105 | |
| 10 μL low retention tips | Sarstedt | 70.1130.105 | |
| pipeteboy 2 | VWR | 612-0927 | |
| 50 mL serological pipette | Appleton Woods | CC117 | |
| 25 mL serological pipette | Appleton Woods | CC216 | |
| 10 mL serological pipette | Appleton Woods | CC214 | |
| glass pipette 270 mm | Fisherbrand | FB50255 | Camera for videos recording |
| pulsin | Polyplus Transfection | 501-04 | Transduction reagent |
| Multitron Standard shaking incubator | Infors HT | INFO28573 | |
| air duster | Office Depot | 1511631 | |
| Name | Company | Catalog Number | Comments |
| WF-NTP Tracker Components and Image Capture Software | |||
| 8'' by 8'' Backlight | Edmond Optics | 88-508 | Tracker component |
| 16 mm FL high resolution lens for 1'' sensor | Edmond Optics | 86-571 | Tracker component |
| 6 Mpx camera | Edmond Optics | 33540 | Tracker component |
| FlyCapture Software | PointGrey | SDK v2.12 | Image capture software |
| WF-NTP Software | Cambridge Enterprise | v1.0 | Image analysis software |
| Office Package | Microsoft | Office 365 | Statistical analysis software |
References
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics. 77 (1), 71-94 (1974).
- Kaletta, T., Hengartner, M. O. Finding function in novel targets: C. elegans as a model organism. Nature Reviews Drug Discovery. 5 (5), 387-399 (2006).
- Nollen, E. A. A., et al. Genome-wide RNA interference screen identifies previously undescribed regulators of polyglutamine aggregation. Proceedings of the National Academy of Sciences of the USA. 101 (17), 6403-6408 (2004).
- Morley, J. F., Brignull, H. R., Weyers, J. J., Morimoto, R. I. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the USA. 99 (16), 10417-10422 (2002).
- Van der Goot, A. T., et al. Delaying aging and the aging-associated decline in protein homeostasis by inhibition of tryptophan degradation. Proceedings of the National Academy of Sciencesof the USA. 109 (37), 14912-14917 (2012).
- Van Ham, T. J., et al. Identification of MOAG-4/SERF as a regulator of age-related proteotoxicity. Cell. 142 (4), 601-612 (2010).
- Van Ham, T. J., et al. C. elegans model identifies genetic modifiers of α-synuclein inclusion formation during aging. PLoS Genetics. 4 (3), e1000027(2008).
- Hamilton, B., et al. A systematic RNAi screen for longevity genes in C. elegans. Genes & Development. 19 (13), 1544-1555 (2005).
- Sarin, S., Prabhu, S., O'Meara, M. M., Pe'er, I., Hobert, O. Caenorhabditis elegans mutant allele identification by whole-genome sequencing. Nature Methods. 5 (10), 865-867 (2008).
- Kim, Y., Sun, H. Functional genomic approach to identify novel genes involved in the regulation of oxidative stress resistance and animal lifespan. Aging Cell. 6 (4), 489-503 (2007).
- Dillin, A., et al. Rates of Behavior and Aging Specified by Mitochondrial Function During Development. Science. 298 (5602), 2398-2401 (2002).
- Lee, S. S., Kennedy, S., Tolonen, A. C., Ruvkun, G. DAF-16 target genes that control C. elegans life-span and metabolism. Science. 300 (5619), 644-647 (2003).
- Jorgensen, E. M., Mango, S. E. The art and design of genetic screens: caenorhabditis elegans. Nature Reviews. Genetics. 3 (5), 356-369 (2002).
- Alavez, S., Vantipalli, M. C., Zucker, D. J. S., Klang, I. M., Lithgow, G. J. Amyloid-binding compounds maintain protein homeostasis during ageing and extend lifespan. Nature. 472 (7342), 226-229 (2012).
- Habchi, J., et al. An anticancer drug suppresses the primary nucleation reaction that initiates the production of the toxic A 42 aggregates linked with Alzheimers disease. Science Advances. 2 (2), e1501244(2016).
- Wu, Y., et al. Amyloid- -Induced pathological behaviors are suppressed by ginkgo biloba extract EGb 761 and ginkgolides in transgenic caenorhabditis elegans. Journal of Neuroscience. 26 (50), 13102-13113 (2006).
- Link, C. D. Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the USA. 92 (20), 9368-9372 (1995).
- Perni, M., et al. A natural product inhibits the initiation of a-synuclein aggregation and suppresses its toxicity. Proceedings of the National Academy of Sciencesof the USA. 114 (6), E1009-E1017 (2017).
- Gidalevitz, T., Krupinski, T., Garcia, S., Morimoto, R. I. Destabilizing protein polymorphisms in the genetic background direct phenotypic expression of mutant SOD1 toxicity. PLoS Genetics. 5 (3), e1000399(2009).
- Nussbaum-Krammer, C. I., Neto, M. F., Brielmann, R. M., Pedersen, J. S., Morimoto, R. I. Investigating the spreading and toxicity of prion-like proteins using the metazoan model organism C. elegans. Journal of visualized experiments. (95), 52321(2015).
- Machino, K., Link, C. D., Wang, S., Murakami, H., Murakami, S. A semi-automated motion-tracking analysis of locomotion speed in the C. elegans transgenics overexpressing beta-amyloid in neurons. Frontiers in Genetics. 5, 202(2014).
- Swierczek, N. A., Giles, A. C., Rankin, C. H., Kerr, R. A. High-throughput behavioral analysis in C. elegans. Nature Methods. 8 (7), 592-598 (2011).
- Van der Goot, A. T., Nollen, E. A. A. Tryptophan metabolism: entering the field of aging and age-related pathologies. Trends in Molecular Medicine. 19 (6), 336-344 (2013).
- Lublin, A. L., Link, C. D. Alzheimer's disease drug discovery: in vivo screening using Caenorhabditis elegans as a model for β-amyloid peptide-induced toxicity. Drug Discovery Today: Technologies. 10 (1), e115-e119 (2013).
- Petrascheck, M., Miller, D. L. Computational analysis of lifespan experiment reproducibility. Frontiers in genetics. 8 (JUN), 92(2017).
- Yemini, E., Jucikas, T., Grundy, L. J., Brown, A. E. X., Schafer, W. R. A database of Caenorhabditis elegans behavioral phenotypes. Nature Methods. 10 (9), 877-879 (2013).
- Wang, S. J., Wang, Z. -W. Track-a-worm, an open-source system for quantitative assessment of C. elegans locomotory and bending behavior. PLoS ONE. 8 (7), e69653(2013).
- Faumont, S., et al. An image-free opto-mechanical system for creating virtual environments and imaging neuronal activity in freely moving Caenorhabditis elegans. PLoS ONE. 6 (9), e24666(2011).
- Tsibidis, G. D., Tavernarakis, N. Nemo: a computational tool for analyzing nematode locomotion. BMC Neuroscience. 8, 86(2007).
- Stirman, J. N., et al. Real-time multimodal optical control of neurons and muscles in freely behaving Caenorhabditis elegans. Nature Methods. 8 (2), 153-158 (2011).
- Leifer, A. M., Fang-Yen, C., Gershow, M., Alkema, M. J., Samuel, A. D. T. Optogenetic manipulation of neural activity in freely moving Caenorhabditis elegans. Nature Methods. 8 (2), 147-152 (2011).
- Restif, C., et al. CeleST: computer vision software for quantitative analysis of C. elegans swim behavior reveals novel features of locomotion. PLoS Computational Biology. 10 (7), e1003702(2014).
- Ramot, D., Johnson, B. E., Berry, T. L., Carnell, L., Goodman, M. B. The Parallel Worm Tracker: a platform for measuring average speed and drug-induced paralysis in nematodes. PLoS ONE. 3 (5), e2208(2008).
- Habchi, J., et al. Systematic development of small molecules to inhibit specific microscopic steps of Aβ42 aggregation in Alzheimer's disease. Proceedings of the National Academy of Sciences of the USA. 114 (2), E200-E208 (2017).
- Aprile, F. A., et al. Selective targeting of primary and secondary nucleation pathways in Aβ42 aggregation using a rational antibody scanning method. Science Advances. 3 (6), e1700488(2017).
- Perni, M., et al. Massively parallel C. elegans tracking provides multi-dimensional fingerprints for phenotypic discovery. Journal of neuroscience. 306, 57-67 (2018).
- Perni, M., et al. Delivery of Native Proteins into C. elegans Using a Transduction Protocol Based on Lipid Vesicles. Scientific Reports. 7 (1), 7380(2017).
- McColl, G., et al. Utility of an improved model of amyloid-beta (Aβ₋) toxicity in Caenorhabditis elegans for drug screening for Alzheimer's disease. Molecular Neurodegeneration. 7 (1), 7380(2012).
- Fatouros, C., et al. Inhibition of tau aggregation in a novel Caenorhabditis elegans model of tauopathy mitigates proteotoxicity. Human Molecular Genetics. 21 (16), 3587-3603 (2012).
- Fay, D. S., Fluet, A., Johnson, C. J., Link, C. D. In vivo aggregation of β-Amyloid peptide variants. Journal of Neurochemistry. 71 (4), 1616-1625 (1998).
- Husson, S. J., Costa, W. S., Schmitt, C., Gottschalk, A. Keeping track of worm trackers. WormBook: the Online Review of C. elegans Biology. , 1-17 (2012).
- Hardaker, L. A., Singer, E., Kerr, R., Zhou, G. T., Schafer, W. R. Serotonin modulates locomotory behavior and coordinates egg-laying and movement in Caenorhabditis elegans. Journal of Neurobiology. 49 (4), 303-313 (2001).
- Tsechpenakis, G., Bianchi, L., Metaxas, D., Driscoll, M. A novel computational approach for simultaneous tracking and feature extraction of C. elegans populations in fluid environments. IEEE Transactions on Bio-medical Engineering. 55 (5), 1539-1549 (2008).
- Buckingham, S. D., Sattelle, D. B. Fast, automated measurement of nematode swimming (thrashing) without morphometry. BMC Neuroscience. 10, 84(2009).
- Feng, Z., Cronin, C. J., Wittig, J. H., Sternberg, P. W., Schafer, W. R. An imaging system for standardized quantitative analysis of C. elegans behavior. BMC Bioinformatics. 5, 115(2004).
- Stroustrup, N., et al. The caenorhabditis elegans lifespan machine. Nature Methods. 10 (7), 665-670 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved