A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
A Whole Body Dosimetry Protocol for Peptide-Receptor Radionuclide Therapy (PRRT): 2D Planar Image and Hybrid 2D+3D SPECT/CT Image Methods
In This Article
Summary
This method estimates the absorbed dose of different structures for peptide-receptor-radionuclide-therapy (PRRT) with the possibility of avoiding organ overlap on 2D-projections. Serial whole-body planar images permit estimation of mean absorbed doses along the whole body, while the hybrid approach, combining planar images and 3D-SPECT/CT image, overcomes the limitations of structure overlapping.
Abstract
Peptide-receptor-radionuclide-therapy (PPRT) is a targeted therapy that combines a short-range energy radionuclide with a substrate with high specificity for cancer cell receptors. After injection, the radiotracer is distributed throughout the entire body, with a higher uptake in tissues where targeted receptors are overexpressed. The use of beta/gamma radionuclide emitters enables therapy imaging (beta-emission) and post-therapy imaging (gamma-emission) to be performed at the same time. Post-treatment sequential images permit absorbed dose calculation based on local uptake and wash-in/wash-out kinetics. We implemented a hybrid method that combines information derived from both 2D and 3D images. Serial whole-body images and blood samples are acquired to estimate the absorbed dose to different organs at risk and to lesions disseminated throughout the body. A single 3D-SPECT/CT image, limited to the abdominal region, overcomes projection overlap on planar images of different structures such as the intestines and kidneys. The hybrid 2D+3D-SPECT/CT method combines the effective half-life information derived from 2D planar images with the local uptake distribution derived from 3D images. We implemented this methodology to estimate the absorbed dose for patients undergoing PRRT with 177Lu-PSMA-617. The methodology could, however, be implemented with other beta-gamma radiotracers. To date, 10 patients have been enrolled into the dosimetry study with 177Lu-PSMA-617 combined with drug protectors for kidneys and salivary glands (mannitol and glutamate tablets, respectively). The median ratio between kidney uptake at 24 h evaluated on planar images and 3D-SPECT/CT is 0.45 (range:0.32-1.23). The comparison between hybrid and full 3D approach has been tested on one patient, resulting in a 1.6% underestimation with respect to full 3D (2D: 0.829 mGy/MBq, hybrid: 0.315 mGy/MBq, 3D: 0.320 mGy/MBq). Treatment safety has been confirmed, with a mean absorbed dose of 0.73 mGy/MBq (range:0.26-1.07) for kidneys, 0.56 mGy/MBq (0.33-2.63) for the parotid glands and 0.63 mGy/MBq (0.23-1.20) for submandibular glands, values in accordance with previously published data.
Introduction
Among peptide-receptor radionuclide therapies, 177Lu-PSMA-617 PRRT combines a short-range beta emitter 177Lu (1.9 mm maximum range in water, half-life 6.71 days) with a prostate-specific membrane antigen (PSMA) ligand. The overexpression of PSMA in 90-100% of local prostate cancer lesions and metastatic disease (lymph node and bone) is the key to this therapy. However, PSMA receptors are also expressed in different healthy tissues where high uptake is often observed during treatments. The main organs at risk are the kidneys, red marrow, salivary and lachrymal glands. The dose to these organs may reduce maximum injectable activity, impairing the therapeutic ratio.
Our institute (IRST IRCCS) activated a protocol with the aim of increasing the therapeutic ratio between lesions and healthy tissues, providing drug protectors combined with 177Lu-PSMA-617 therapy. Mannitol, polyglutamate folate tablets combined with externally applied ice packs and N-acetylaspartylglutammate acid eye drops are used for kidneys, salivary and lachrymal gland preservation, respectively1. Post-infusion dosimetric studies are required to estimate the effective half-life (i.e., combination of physical and biological half-life) and absorbed dose for different structures of interest localized throughout the body (e.g., kidneys, salivary glands, disseminated lesions). This scenario requires whole body information obtained by acquiring sequential post-infusion whole-body planar images2. However, the overlap of high uptake structures (e.g., transient intestine uptake above the kidneys) requires 3D information capable of discriminating between different local uptakes that are blended on 2D projections. We implemented a hybrid method capable of providing a dosimetric evaluation of the entire body thanks to 2D planar images2, maintaining 3D information on a selected region (e.g., abdominal region). This method combines the activity distribution provided by 3D SPECT/CT images with the effective half-life calculated from planar images. Information obtained from other non-overlapping structures (e.g., salivary glands) are derived from planar image study only. The blood sample method used for red marrow evaluation is described in another section.
The advantage of the hybrid approach is that the entire body can be scanned, whereas a full 3D SPECT/CT method limits cranio-caudal image extension, which may make it impossible to study structures that are distant from each other. However, the low image resolution of planar imaging and the need to implement an overlap correction using a single 3D SPECT/CT acquisition represent the main drawbacks.
In order to test the safety and efficacy of PRRT therapies, it is important to compare single institution data with data previously published by other groups. The majority of published data with 177Lu-PSMA-617 are based on planar images. Thus, the described method could also be useful for the standardization of the methodologies used. Finally, it is worthy of note that the implementation of the methodology requires a high degree of collaboration between different professional figures involved (i.e., physicians, physicists, medical radiology technicians, nurses).
Protocol
The dosimetry procedure was performed according to the treatment protocol "Radiometabolic Therapy (RMT) with 177Lu-PSMA-617 in advanced castration resistant prostate cancer (CRPC): efficacy and toxicity evaluation" (EUDRACT/RSO number: 2016-002732-32) (Figure 1). Selected patients underwent dosimetry evaluation based on performance status. All patients signed informed consent. Prior to treatment delivery, each patient underwent a 68Ga-PSMA-11 PET/CT whole body scan.
NOTE: It is important to underline that some steps are linked specifically to the scanner used.
1. Pre-infusion Imaging: Transmission and Blank Image Acquisition
NOTE: In this first image acquisition the patient's water equivalent thickness is evaluated. This value is used for attenuation correction of counts derived from 2D planar images acquired post 177Lu-PSMA-617 injection.
- Set low energy high resolution collimators (LEHR).
- Open the image protocol acquisition on the workstation and select transmission scan whole body planar image acquisition.
- Check the table velocity (e.g., 7 cm/min) and zoom (e.g., 1). Keep these values equal for the blank scan acquisition. Check that the option Body Contour is disabled.
- Position the patient on the couch feet-first supine with arms at-rest along the side of the body. Use this position for all the images. If necessary, use available supports (arm support, knee wedge, pillow, blanket).
- Take note of the exact position of the patient, using the scale number along the couch: vertex head position, knee position, foot position, couch height, all supports used. Take note of the patient's weight and height.
- Set the SPECT dual heads at the opposite positions (i.e., 0° and 180°) and at the maximum distance from the FOV center. Raise the couch so that the patient is positioned at the FOV center and with head at the detector center.
- Position the 57Co flood support on the posterior camera and then the 57Co flood itself on the support. Start image acquisition.
- At the end of image acquisition, remove the 57Co flood and support. Press Unload on the teach pendant. Help the patient to get up.
- Repeat the image acquisition in the same way but without the patient positioned on the couch.
NOTE: Couch velocity, table height and camera distance should be set at the same value as the previous transmission image.
2. Post-infusion Image Acquisition: Planar Image
NOTE: Planar post-image acquisitions are used for effective half-life and mean absorbed dose evaluation of different structures.
- Acquire first image 0.5-1 h after 177Lu-PSMA-617 infusion (day 1, Figure 1).
- Acquire the first image before bladder voiding. If the patient feels an urgent need for bladder voiding, provide a proper vessel for urine collection. Take care to include the vessel (or urine bag if the patient has a catheter) in the image.
- Collect a 2 mL blood sample, close the collection tube and place it in a shielded box, noting the time.
- Change to medium energy high resolution collimator (MEHR).
- Open the image protocol acquisition on the workstation and select whole body planar image acquisition. Check the table velocity (e.g., 7 cm/min) and zoom (e.g., 1). Keep these values equal for all the other images. Check that the option Body Contour is disabled.
- Position the patient on the couch, ensuring that the position is the same as that used for the previous image (i.e., pre-infusion transmission scan).
- Set the SPECT dual heads at opposite positions (i.e., 0° and 180°). Raise the table so that the patient is positioned at the FOV center and with the head at the detector center.
- Using the teach pendent, manually adjust the position of the posterior camera (i.e., positioned at 180°) to reach the minimum distance from the inferior couch profile.
- Manually adjust the position of anterior camera (i.e., positioned at 0°) to reach minimum distance from the patient's profile. Take into account the entire body surface along the whole patient height to avoid collision during scanning.
- Taking note of the position of the duel heads, start image acquisition.
- At the end of image acquisition, press Unload on the teach pendant and help the patient to get up.
- Repeat the same image acquisition with the same camera settings at 16-24 h (second image, day 2), 36-48 h (third image, day 3). Additional images (one or more) can be acquired up to 120 h post infusion (e.g. 66-70 h and 120 h) based on patient compliance and Institution resources.
- Collect a 2 mL blood sample at the same time as the SPECT image acquisition, close the collection tube and place it in a shielded box, making a note of the time.
3. Post-infusion Image Acquisition: 3D SPECT/CT
NOTE: On day 2 (16-24 h post infusion) a 3D image acquisition is performed, together with the planar image acquisition. The 3D SPECT/CT image focuses on the abdominal region and enables organ overlap (e.g., kidneys or intestinal loops) to be avoided on anterior/posterior projections.
- After planar image acquisition, select the 3D SPECT/CT image inside the dosimetry protocol on the workstation.
- Check that the proper image parameters have been set: acquisition modality (e.g., step-and-shoot), angle per projection (e.g., 5°), number of frames per rotation (e.g., 72), frame duration (e.g., 3,000 ms). Check that Body Contour is disabled.
- Position the detector at the maximum distance from the center to avoid collision. Position the patient with arms lifted over the head. Position the patient table inside the camera until when the desired region is centered on the detector (e.g., kidneys and a specific lesion situated in the same region). Start image acquisition.
- Acquire the corresponding CT image.
- At the end of image acquisition, press Unload on the teach pendant and help the patient get up.
4. Image Analysis
NOTE: Scatter, attenuation, and background corrections are implemented. Single organ and lesion mass are considered for absorbed dose evaluation. ROI and VOI are contoured on planar and 3D images.
- Send all acquired images from the acquisition workstation to the analysis workstation.
- For all post-infusion images, select emissive, low and high scatter images and click on the right panel of the dedicated workflow to create a scatter corrected image
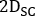 , as follows:
, as follows:
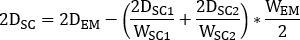
where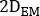 ,
, 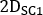 and
and 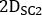 are emissive, lower scatter and higher scatter 2D anterior or posterior planar whole-body images, respectively;
are emissive, lower scatter and higher scatter 2D anterior or posterior planar whole-body images, respectively; 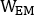 ,
, 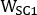 and
and 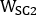 are emissive, lower scatter and higher scatter energy window widths, respectively.
are emissive, lower scatter and higher scatter energy window widths, respectively. - Open each posterior image, click on Image, then Reorient, Pan, Zoom..., flag Y mirror, click Apply & Quit, and then save the rotated left-right image.
- Open anterior and posterior (rotated) scatter-corrected planar images acquired post infusion.
- Select the image acquired on day 2 as the most suitable for ROI delineation. Contour organs: whole body (encompassing also urine vessel or bag when needed), kidneys, liver, spleen (if visible), parotid glands, submandibular glands, lachrymal glands. If possible, also contour some visible lesions. Contour the ROIs on the most useful image between anterior and posterior views (Figure 2). Contour a small ROI adjacent to each contoured structure for background.
- Copy and paste all ROIs from the image acquired on day 2 to the anterior and posterior views of the other images acquired post infusion.
- Use only ROI translation and do not modify to maintain the same organ dimension. For each acquired post infusion, select anterior image. Save contoured ROIs.
- For each image, take note of average counts [c] and pixel dimension inside each ROI (including background ROIs) for both anterior and posterior views3.
- Open anterior transmission and blank scans, together with delineated ROIs. Copy and paste organ and lesions ROIs onto transmission scan. Adjust for organ mismatch, and if needed, enlarge or decrease organ contours for different image magnification.
- For body attenuation, contour a structure encompassing head, shoulders, chest and abdomen, avoiding arms and legs (Figure 3).
- Copy and paste all ROIs from transmission to blank scan.
- Evaluate the water equivalent thickness z for each structure to estimate the self-attenuation. Take note of average counts inside each ROI on both transmission (Itransmission) transmission and blank (Iblank) scans. Calculate water equivalent thickness z as
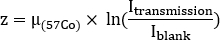
where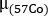 is the attenuation coefficient for 57Co flood previously measured with a uniform phantom.
is the attenuation coefficient for 57Co flood previously measured with a uniform phantom. - Use the pre-treatment 68Ga-PSMA-11 PET/CT scan. Contour organs on CT image: kidneys, liver, spleen, parotid glands and submandibular glands. Contour lesions on PET images. Assuming a uniform water composition for each structure, calculate the mass of each contoured structure using a unit density (1 g/mL).
- Perform SPECT/CT image reconstruction, taking into account scatter correction, CT attenuation correction and resolution recovery. Set the same iterative reconstruction values as used for SPECT calibration (e.g., OSEM iteration and subset numbers, post reconstruction filtering).
5. Blood Sample Measurements
NOTE: Blood sample measurements are performed on High Purity Germanium (HPGe) detector for red marrow dose estimation.
- Let blood sample decay for approximately 2 weeks to avoid detector saturation and high dead time.
- After 2 weeks, measure one sample at a time. Because of the low activity, start measurements from the last acquired blood sample (i.e., from day 6).
- Position the blood sample collection tube on the dedicated holder. Use the same geometry as that used for HPGe calibration. Position it on the HPGe detector and close the detector shielding case.
- Open the software for spectrum acquisition and analysis. Check that the dead time is <3%. If higher, wait a few more days and perform the measurements then.
- Select the proper HPGe calibration file corresponding to the 2 mL collection tube geometry holder. Start sample measurements (minimum 12 h measurements).
- Analyze the spectrum by identifying the mean gamma peak and by calculating activity concentration. Take note of both measured sample activity and time and date measurements.
- Repeat the same measurements and analysis for all of the blood samples.
6. Dosimetry evaluation
NOTE: The analysis is performed with a dedicated dosimetry software based on MIRD publications4,5,6,7,8. For each considered structure, effective half-life is evaluated on sequential 2D whole body images by bi- or mono-exponential curve fitting of time-activity curves. 3D SPECT/CT imaging is used to resolve the problem of high uptake intestine overlap on kidney structure by scaling the time-activity curves derived from planar images. Mean absorbed dose is then calculated for each structure mass. For red marrow dose evaluation, blood samples measurements are used and scaled to the patient's weight.
- Planar images
- For each image and structure, calculate the counts on anterior (
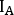 ) and posterior (
) and posterior (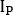 ) view as
) view as
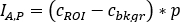
where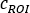 is the average count [c] for the considered ROI,
is the average count [c] for the considered ROI, 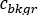 is the average count [c] in the corresponding background region, and
is the average count [c] in the corresponding background region, and 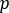 is the pixel number inside the ROI.
is the pixel number inside the ROI. - For each ROI, calculate the uptake at each image time point as
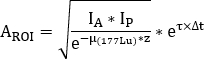
where is the attenuation correction factor for 177Lu,
is the attenuation correction factor for 177Lu,  is the 177Lu physical half-life, Δt is the time difference between infusion and image acquisition9, and z is the water equivalent thickness evaluated on transmission scan.
is the 177Lu physical half-life, Δt is the time difference between infusion and image acquisition9, and z is the water equivalent thickness evaluated on transmission scan. - Calculate the relative uptake as
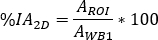
where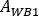 is
is 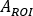 evaluated for whole body on the first post-infusion image. As whole urine is included in the image, this is considered as a reference for the total effective infused activity.
evaluated for whole body on the first post-infusion image. As whole urine is included in the image, this is considered as a reference for the total effective infused activity.
- For each image and structure, calculate the counts on anterior (
- Hybrid 2D+3D SCPET/CT images
- For SPECT/CT activity calibration, image a cylindrical phantom with a central sphere of known activity. Contour the central sphere VOI and calculate the calibration factor [cps/MBq] as
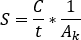
where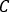 are the total counts inside the VOI [c],
are the total counts inside the VOI [c],  the image acquisition time [sec] and
the image acquisition time [sec] and  the known injected activity [MBq] inside the central sphere. SPECT/CT image for the patient is performed with the same acquisition and reconstruction parameter settings.
the known injected activity [MBq] inside the central sphere. SPECT/CT image for the patient is performed with the same acquisition and reconstruction parameter settings. - Open the SPECT/CT image. Contour volumes of interest (VOIs) (e.g., kidneys, visible lesion) are based on both uptake information and CT morphology. Calculate the activity in the structure as
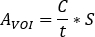
- Calculate
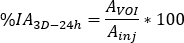
where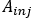 is the injected activity during treatment.
is the injected activity during treatment. - Calculate the scaling factor for the time activity curve as

where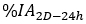 is the
is the 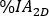 calculated on planar image on day 2 (16-24 h) decay-corrected for physical half life at the time of injection.
calculated on planar image on day 2 (16-24 h) decay-corrected for physical half life at the time of injection. - Rescale the kidney 2D time activity curve
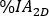 with
with 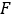 factor accordingly. Perform dosimetry evaluation with OLINDA/EXM as described below.
factor accordingly. Perform dosimetry evaluation with OLINDA/EXM as described below.
- For SPECT/CT activity calibration, image a cylindrical phantom with a central sphere of known activity. Contour the central sphere VOI and calculate the calibration factor [cps/MBq] as
- Adult male phantom
- Open dosimetry software. Select the radionuclide (e.g., 177Lu) inside the Nuclide Input Form module. Select the model (e.g., Adult Male) inside the Model Input Form module.
- Go to the Kinetic Input Form module and click Clear All Data. Click on Fit to Model and a separate window will open.
- In the Time (Hr) column, insert the hours post infusion for each image acquisition, in hour format (e.g., 1 h and 30 min will be 1.50). Scroll down the organ menu and select organs of interest (e.g., kidneys, liver, spleen).
- For each organ, insert the relative uptake
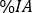 at each image time point. Click Refresh.
at each image time point. Click Refresh. - For paired organs (i.e., kidneys) insert a single value as sum of left and right single relative uptakes
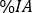 . Click Refresh and check the point distributions on the left-end side plot.
. Click Refresh and check the point distributions on the left-end side plot. - Perform a curve fitting using an exponential curve as
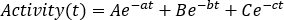
A, B and C parameters may assume positive or negative values for wash-in and wash-out phase modeling, respectively. If data of time activity curves are decay-corrected, a, b and c parameters represent biological half life λbiol and are all positive. Choose an appropriate curve-fitting model between mono, bi or tri-exponential curves. Flag the required parameters, insert starting values and click Fit until the fit is performed. - Take note of curve-fitting parameters. Calculate effective half-life as
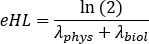
where λphys is the physical half life of 177Lu, and λbiol is the biological half life of 177Lu-PSMA-617 compound. For λbiol, consider the lowest values among a, b and c curve-fitting parameters (i.e., corresponding to the higher effective half life). - Repeat from step 6.3.3 to step 6.3.7. for each organ.
- Insert the relative uptake at each image time point for the remainder of the body (namely Total Body/Rem Body) by subtracting the relative uptake of all considered organs from the whole-body uptake. Repeat from step 6.3.5 to step 6.3.7 for Total Body/Rem Body. Generally, a bi-exponential curve fitting is recommended.
- Click Done and save the model. The program goes back to the Kinetic Input Form module and the number of disintegrations per unit of injected activity (namely ND, expressed in Bq*h/Bq) is visualized for each considered organ.
- Go to Main Input Form. Click on Doses, and then Modify Input Data. In the box at the bottom Multiply all masses by:, insert the ratio between the patient's weight and Adult Male phantom weight (i.e., 73.7 kg). Click on the Multiply all masses by: button. All organ masses will be then rescaled accordingly. Insert single organ masses as calculated from CT delineation for the analyzed organs. For paired organs such as kidneys, insert the sum of left and right kidney masses. Click Done.
- The report will display the mean absorbed dose normalized to injected activity, expressed in mGy/MBq. Take note of the total absorbed dose for considered organs (i.e., kidneys, liver, spleen, and Total Body).
- Repeat for time activity curves derived from the hybrid 2D+3D SPECT/CT method.
- Red marrow
- Perform scaling for blood values to calculate Red Marrow dose.
- Calculate the blood uptake at each blood sample acquisition as
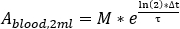
where M is the activity measurement [MBq] obtained with HPGe 2 mL blood sample measurement. - Calculate the blood relative uptake
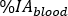 as
as
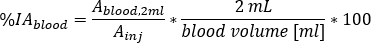
where blood volume [mL] is the total blood volume estimation for the specific patient. This value is taken from the Adult Male standard phantom values10. - Rescale to Red Marrow (RM) mass and calculate the RM relative uptake
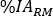 as
as
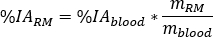
where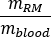 is the ratio of standard Adult Male phantom of
is the ratio of standard Adult Male phantom of 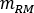 (Red Marrow mass) equal to 1120 g and
(Red Marrow mass) equal to 1120 g and 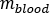 (whole body blood mass) equal to 5000 g.
(whole body blood mass) equal to 5000 g. - Go to Kinetic Input Form module and click Clear All Data. Click on Fit to Model. Scroll down the organ menu and select Red Marrow.
- In the Time (Hr) column, insert the hours post infusion for each blood sample acquisition in hour format (i.e., 1 h and 30 min will be 1.50). Insert the values of
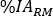 . Repeat steps 6.3.5-6.3.7. for Red Marrow.
. Repeat steps 6.3.5-6.3.7. for Red Marrow. - Scroll down the organ menu and select Total Body/Rem Body. In the Time (Hr) column, insert the hours post infusion for each image acquisition in hour format (i.e., 1 h and 30 min will be 1.50). Insert the values of
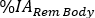 equal to the difference between
equal to the difference between 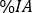 of whole body calculated on planar images and
of whole body calculated on planar images and 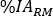 .
. - Repeat from step 6.3.5 to point for Red Marrow.
- Click Done and save the Model.
NOTE: The program goes back to the Kinetic Input Form module and the number of disintegrations per unit of injected activity (namely ND, expressed in Bq*h/Bq) is visualized for each considered. - Go to Main Input Form. Click on Doses. Scale organ mass rescaling as the previous analysis on other organs.
- Sphere model
- Use a unit density sphere model for structures that are not available in the phantom (e.g., lesions, parotid and submandibular glands).
- For curve fitting, repeat from step 6.3.2 to step 6.3.10, substituting organ values with relative uptake for separated salivary glands and lesions.
- Click Done and save the model.
- The program goes back to the Kinetic Input Form module and the number of disintegrations per unit injected activity [Bq*h/Bq] is visualized for each considered organ. Take note of ND for each considered structure.
- Go to Model Input Form. Click on Spheres.
- For each structure, enter the calculated ND. Click on Calculate Doses. The report will display the mean absorbed dose normalized to injected activity, expressed in mGy/MBq, for discrete increasing sphere masses (g). Fit the curve with mono-exponential fitting and calculate the absorbed dose normalized to injected activity (mGy/MBq) for the specific structure mass.
- For paired organs (e.g., salivary glands), perform the sphere model evaluation separately for left and right organs. Use the mean value between left and right structure for whole organ dose evaluation.
Results
Dosimetry was performed for 10 patients (7 undergoing first treatment cycle, 3 second cycle). Blood samples were acquired from all but 3 patients. One patient voided the bladder before the first post-infusion image acquisition. Injected activity was 5.5 GBq for 5 patients and 4.4 GBq for 5 patients.
With regard to curve fitting, mono or bi-exponential curve fitting was used for organ time-activity-curves. Bi-exponential cu...
Discussion
The method described enables whole body dosimetry to be performed for PRRT therapies and is a valid compromise between 2D whole-body and 3D dosimetry information in that it provides valuable information without significantly increasing image acquisition load. The method is also useful for the evaluation of the absorbed dose of overlapping structures and provides information on the structures lying outside the 3D SPCET/CT limited field of view.
The implementation of the methodology requires a h...
Disclosures
The authors have nothing to disclose.
Acknowledgements
Our thanks go to the professional figures involved in the protocol (i.e., physicians, physicists and nurses) and to the patients who agreed to take part in the study. We are also grateful to the medical radiology technicians of the Nuclear Medicine Unit for their help with protocol implementation: Valentina Mautone, Maria Caternicchia, Monia Pancisi, Daniela Fichera and Delia Bevilacqua. The authors acknowledge Alessandro Savini and Simone Marzoni for their help in the video recording. The work was partially supported by AIRC (Italian Association for Cancer Research, grant number: L2P1367 - L2P1520). The work was partially financed by the Italian Minister of Health.
Materials
| Name | Company | Catalog Number | Comments |
| 177Lu EndolucinBeta | ITG - Isotopen Technologien München AG, Lichtenbergstrasse 1, 85748 Garching, Germany, info@itm.ag | Radiotracer 177Lu for therapy purpuse | |
| Biograph mCT Flow PET/CT | Siemens Healthineers, Erlangen, Germany | PET/CT scanner | |
| C-Thru 57Co planar flood - Model MED3709 | Eckert & Ziegler, Strahlen- und Medizintechnik AG, Robert-Rössle-Str. 10, 13125 Berlin, Germany, info@ezag.de | Calibration/planar source | |
| Cylindrical phantom with spheric insert | Data Spectrum Corporation, 1605 East Club Boulevard, Durham NC 27704-3406, US, info@spect.com | Phantom for SPECT/CT calibration | |
| Discovery NM/CT 670 SPECT/CT | International General Electric, General Electric Medical System, Haifa, Israel | SPECT/CT scanner | |
| GalliaPharm 68Ge/68Ga Generator | Eckert & Ziegler, Strahlen- und Medizintechnik AG, Robert-Rössle-Str. 10, 13125 Berlin, Germany, info@ezag.de | 68Ge/68Ga Generator of 68Ga for imaging purposes | |
| GammaVision v 6.08 | Ortec, Ametek - Advanced Measurement Technology, 801 South Illinois Avenue, Oak Ridge, Tennessee 37830, US, ortec.info@ametek.com | Gamma Spectorscopy software | |
| High Purity Germanium HPGe, model GEM30P4-70 | Ortec, Ametek - Advanced Measurement Technology, 801 South Illinois Avenue, Oak Ridge, Tennessee 37830, US, ortec.info@ametek.com | Gamma spectometer | |
| MimVista Software | MIM Software INC, Cleveland, OH 44122, US | Workstation | |
| OLINDA/EXM v 1.1 | RADAR - RAdiation Dose Assessment Resource, West End Ave, Nashville, TN 37235, US (now commercially available as OLINDA/EXM v 2.0, Hermes Medical Solutions, Strandbergsgatan 16, 112 51 Stockholm, Sweden, info@hermesmedical.com) | Dosimetry software | |
| PSMA 11 | ABX advanced biochemical compounds - Biomedizinische,Heinrich-Gläser-Straße 10-14, 01454 Radeberg, Germania, info@abx.de | Carrier for 68Ga radiotracer | |
| PSMA 617 | Endocyte Inc. (Headquarters), 3000 Kent Avenue, West Lafayette, IN 47906 | Carrier for 177Lu radiotracer | |
| Xeleris4.0 | International General Electric, General Electric Medical System, Haifa, Israel | Workstation |
References
- Matteucci, F., et al. Reduction of 68Ga-PSMA renal uptake with mannitol infusion: preliminary results. European Journal of Nuclear Medicine and Molecular Imaging. , 1-6 (2017).
- Sarnelli, A., et al. Dosimetry of 177 Lu-PSMA-617 after mannitol infusion and glutamate tablet administration: Preliminary results of EUDRACT/RSO 2016-002732-32 IRST protocol. Molecules. 24 (3), (2019).
- Stabin, M. G. . Fundamentals of nuclear medicine dosimetry. , (2008).
- Snyder, W. S., Ford, M. R., Warner, G. G., Watson, S. B. MIRD Pamphlet No. 11: "S" Absorbed dose per unt cumulate activity for selected radionuclides and organs. Society of Nuclear Medicine. , (1975).
- Bolch, W. E., et al. MIRD Pamphlet No. 17: The Dosimetry of Nonuniform Activity Distributions-Radionuclide S Values at the Voxel Level. Journal of Nuclear Medicine. 40 (17), 11s-36s (1998).
- Stabin, M. G., Sparks, R. B., Crowe, E. OLINDA/EXM: The Second-Generation Personal Computer Software for Internal Dose Assessment in Nuclear Medicine. Journal of Nuclear Medicine. 46, 1023-1027 (2005).
- Hippeläinen, E., Tenhunen, M., Mäenpää, H., Heikkonen, J., Sohlberg, A. Dosimetry software Hermes Internal Radiation Dosimetry: from quantitative image reconstruction to voxel-level absorbed dose distribution. Nuclear Medicine Communications. 38 (5), 357-365 (2017).
- Stabin, M. G., Siegel, J. A. RADAR Dose estimate report: a compendium of radiopharmaceutical dose estimates based on OLINDA/EXM version 2.0. Journal of Nuclear Medicine. 59, 154-160 (2018).
- Siegel, J., et al. MIRD pamphlet no. 16: Techniques for quantitative radiopharmaceutical biodistribution data acquisition and analysis for use in human radiation dose estimates. Journal of Nuclear Medicine. 40 (2), 37S-61S (1999).
- Valentin, J. Basic anatomical and physiological data for use in radiological protection: reference values. Annals of ICRP. 32, 5 (2002).
- Frey, E. C., Humm, J. L., Ljungberg, M. Accuracy and precision of radioactivity quantification in nuclear medicine images. Seminars in Nuclear Medicine. 42 (3), 208-218 (2012).
- Violet, J. A., et al. Dosimetry of Lu-177 PSMA-617 in metastatic castration-resistant prostate cancer: correlations between pre-therapeutic imaging and "whole body" tumor dosimetry with treatment outcomes. Journal of Nuclear Medicine. , (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved