A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Creating Highly Specific Chemically Induced Protein Dimerization Systems by Stepwise Phage Selection of a Combinatorial Single-Domain Antibody Library
In This Article
Summary
Creating chemically induced protein dimerization systems with desired affinity and specificity for any given small molecule ligand would have many biological sensing and actuation applications. Here, we describe an efficient, generalizable method for de novo engineering of chemically induced dimerization systems via the stepwise selection of a phage-displayed combinatorial single-domain antibody library.
Abstract
Protein dimerization events that occur only in the presence of a small-molecule ligand enable the development of small-molecule biosensors for the dissection and manipulation of biological pathways. Currently, only a limited number of chemically induced dimerization (CID) systems exist and engineering new ones with desired sensitivity and selectivity for specific small-molecule ligands remains a challenge in the field of protein engineering. We here describe a high throughput screening method, combinatorial binders-enabled selection of CID (COMBINES-CID), for the de novo engineering of CID systems applicable to a large variety of ligands. This method uses the two-step selection of a phage-displayed combinatorial nanobody library to obtain 1) "anchor binders" that first bind to a ligand of interest and then 2) "dimerization binders" that only bind to anchor binder-ligand complexes. To select anchor binders, a combinatorial library of over 109 complementarity-determining region (CDR)-randomized nanobodies is screened with a biotinylated ligand and hits are validated with the unlabeled ligand by bio-layer interferometry (BLI). To obtain dimerization binders, the nanobody library is screened with anchor binder-ligand complexes as targets for positive screening and the unbound anchor binders for negative screening. COMBINES-CID is broadly applicable to select CID binders with other immunoglobulin, non-immunoglobulin, or computationally designed scaffolds to create biosensors for in vitro and in vivo detection of drugs, metabolites, signaling molecules, etc.
Introduction
CID systems, in which two proteins dimerize only in the presence of a small-molecule ligand (Figure 1), offer versatile tools for dissecting and manipulating metabolic, signaling, and other biological pathways1. They have demonstrated the potential in biological actuation, such as drug-controlled T cell activation2 and apoptosis3,4, for improving the safety and efficacy of adoptive T cell therapy. Additionally, they provide a new methodology for in vivo or in vitro detection of small-molecule targets. For example, CID proteins can be genetically fused with fluorescence reporter systems (e.g., fluorescence resonance energy transfer (FRET)5 and circularly permuted fluorescent proteins)6 for real-time in vivo measurements, or serve as affinity reagents for sandwich enzyme-linked immunosorbent assay (ELISA)-like assays.
Despite their wide applications, creating new CID systems that can be controlled by a given small-molecule ligand has major challenges. Established protein binder engineering methods including animal immunization7, in vitro selection8,9, and computational protein design10 can generate ligand binding proteins that function via binary protein-ligand interactions. However, these methods have difficulties creating a ligand-induced ternary CID complex. Some methods create CID by chemically linking two ligands that independently bind to the same or different proteins11,12,13,14,15,16 or rely on selecting binder proteins such as antibodies targeting preexisting small molecule-protein complexes17,18, and thus have a limited choice of ligands.
We recently developed a combinatorial binders-enabled selection of CID (COMBINES-CID) method for de novo engineering of CID systems19. This method can obtain the high specificity of ligand-induced dimerization (e.g., an anchor-dimerization binder dissociation constant, KD (without ligand)/KD (with ligand) > 1,000). The dimerization specificity is achieved using anchor binders with flexible binding sites that can introduce conformational changes upon ligand binding, providing a basis for the selection of conformationally selective binders only recognizing ligand-bound anchor binders. We demonstrated a proof-of-principle by creating cannabidiol (CBD)-induced heterodimers of nanobodies, a 12–15 kDa functional antibody fragment from camelid comprising a universal scaffold and three flexible CDR loops (Figure 2)20, which can form a binding pocket with adaptable sizes for small-molecule epitopes21,22. Notably, the in vitro selection of a combinatorial protein library should be cost-effective and generalizable for CID engineering because the same high-quality library can be applied to different ligands.
In this protocol and video, we focus on describing the two-step in vitro selection and validation of anchor (Figure 3A) and dimerization binders (Figure 3B) by screening the combinatorial nanobody library with a diversity higher than 109 using CBD as a target, but the protocol should be applicable to other protein libraries or small-molecule targets. The screening of CID binders usually takes 6–10 weeks (Figure 4).
Access restricted. Please log in or start a trial to view this content.
Protocol
1. Library construction
- Use a synthetic combinatorial single-domain antibody library with a diversity of ~1.23–7.14 x 109, as previously described19. While this protocol does not include library construction, it can be applied to other combinatorial binder libraries.
2. Biotinylation of ligand target or ligand
- Biotinylate the selected ligand, for example, CBD and tetrahydrocannabinol (THC)19, via various chemical synthesis strategies, depending on the suitable biotinylation sites of a target.
3. Anchor binder screening
- Beginning of selection
- Begin every round of selection by inoculating a single TG1-cell colony, freshly grown in 6 mL of 2YT at 37 ˚C and 250 revolutions per minute (rpm) to a 600 nm (OD600) absorbance of ~0.5. Incubate the cells on ice for the use in step 3.5.1.
- Negative selection with biotin-bound streptavidin beads
- Prepare the "negative selection beads" by washing 300 µL of streptavidin-coated magnetic beads using a magnetic separation rack, 3x with 0.05% phosphate-buffered saline with Tween buffer (PBST, 1 x PBS with 0.05% vol/vol Tween 20%) and 2x with 1 x PBS.
- Resuspend the beads with 1 mL of 1% casein in 1 x PBS (pH = 7.4), and saturate the beads by adding 5x the reported binding capacity using biotin. Incubate at room temperature (RT) on a rotator for 1 h.
- Wash the beads 5x using 0.05% PBST and 3x using 1 x PBS, for a total of eight washes.
- Add ~1013 phage particles in 1% casein/1% BSA in 1 x PBS (pH = 7.4) and incubate at RT on a rotator for 1 h.
- After incubation, collect the supernatant to be used in step 3.3.6.
- Positive selection with biotinylated ligand-bound streptavidin beads
- Prepare the "positive selection beads" using 1/2 the volume of the beads used for the "negative selection beads" following steps 3.2.1.
- Re-suspend the beads with 1 mL 1% casein in 1 × PBS, pH 7.4 and saturate the beads by adding 5x the full binding capacity calculated based on the manual using the biotinylated ligand of choice. Incubate at RT on a rotator for 1 h.
- Wash the beads 5x using 0.05% PBST and 3x using 1 x PBS, for a total of eight washes.
- Block the beads with 1 mL of 1% casein/1% BSA in 1 x PBS (pH = 7.4) and incubate at RT on a rotator for 1 h to prevent nonspecific binding between the phages and the streptavidin-coated magnetic beads.
- Wash the streptavidin-coated magnetic beads 3x using 0.05% PBST and one time using 1 x PBS, for a total of four washes.
- Resuspend the streptavidin-coated magnetic beads using the unbound phages taken from step 3.2.5 and incubate at RT on a rotator for 1 h.
- Extract the supernatant without disturbing the magnetic beads. Save the unbound phages as input, to be used in step 3.5.1.
- Wash the beads 10x using 0.05% PBST and 5x using 1 x PBS. In between every three washes transfer them to a new tube to avoid phages nonspecifically bound to the tube walls.
- Elution of phage-displayed nanobodies
- Competitively elute bound phages by adding 450 µL of the non-biotinylated ligand, using a concentration in the micromolar range (e.g., 10–50 µM) and incubating at RT on a rotator for 30 min. The selected ligand concentration for the competitive elution of bound phages is dependent on desired KD of the "anchor binder". Ligand concentrations can be relatively high in initial selection rounds and then decreased in later rounds.
- Collect supernatant and save the eluted phages as output, to be used in step 3.5.2.
- Input/output titrations and infection
- For input titration, prepare 10x serial dilutions in 1 x PBS up to 109-fold with the input phage from step 3.3.7. Use the 107–109 serial dilutions to do infections by transferring 10 µL input phage from each dilution to 70 µL TG1 cells (OD600 of ~0.5). Incubate at 37 ˚C for 45 min, plate the infected TG1 cells on three 90 mm 2YT-agar dishes containing 100 μg/mL ampicillin and 2% (wt/vol) glucose, and incubate overnight at 37 ˚C. From the overnight plates, phage input can be calculated as follows:
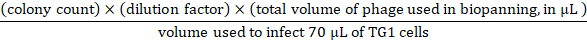
- For output infection and titration, transfer the eluted phages from step 3.4.2 to 3 mL of TG1 cells (OD600 of ~0.5). Incubate in a water bath at 37 ˚C for 45 min. Then prepare 10x serial dilutions in 2YT up to 103-fold, plate each dilution on 90 mm 2YT-agar dishes, and incubate overnight at 37 ˚C. From the overnight plates, phage output can be calculated as follows:
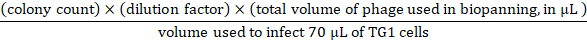
- Divide the remaining infected TG1 cells on three 150 mm 2YT-agar plates containing 100 μg/mL ampicillin and 2% (wt/vol) glucose. Incubate plates overnight at 37 ˚C.
- For input titration, prepare 10x serial dilutions in 1 x PBS up to 109-fold with the input phage from step 3.3.7. Use the 107–109 serial dilutions to do infections by transferring 10 µL input phage from each dilution to 70 µL TG1 cells (OD600 of ~0.5). Incubate at 37 ˚C for 45 min, plate the infected TG1 cells on three 90 mm 2YT-agar dishes containing 100 μg/mL ampicillin and 2% (wt/vol) glucose, and incubate overnight at 37 ˚C. From the overnight plates, phage input can be calculated as follows:
- Library amplification and recovery for further rounds of selection
- Add 3 mL of 2YT per plate, scrape with a sterile cell scraper and collect all cells in a 50 mL conical tube. Mix the collected cells with sterile glycerol (20% wt/vol final concentration). Measure the OD600 of the mixture and make 3–5 stock aliquots. Store at -80 ˚C for long-term storage.
- For phage rescue, dilute the phagemid-containing TG1 bacterial mixture using 25 mL of 2YT media supplemented with 2% glucose and 100 μg/mL ampicillin to an OD600 of ~0.1. Culture cells at 37 ˚C and 250 rpm to an OD600 of ~0.5.
- Superinfect the cells by adding CM13 helper phage at 5 x 109 pfu/mL and incubate at 37 ˚C and 250 rpm for 45 min. The CM13 helper phage provides required phage coat proteins for the assembly of complete phage particles.
- Centrifuge the culture at 8,000 x g for 10 min to remove the glucose. Resuspend the cells using 50 mL of 2YT media supplemented with 100 μg/mL ampicillin and 50 μg/mL kanamycin and incubate at 25 ˚C and 250 rpm overnight.
- Centrifuge the cells from the overnight culture at 9,000 x g, 4 ˚C for 30 min. Transfer supernatant to a new tube and precipitate phages in the supernatant using 1/5 volume PEG/NaCl solution (20% wt/vol polyethylene glycol-6,000 and 2.5 M NaCl). Mix gently and place on ice for 1 h.
- Collect phage particles by centrifugation using 12,000 x g at 4 ˚C for 30 min. Resuspend the pellets using 1 mL of 1 x PBS, and transfer the suspension to a microcentrifuge tube. Centrifuge the tube at 20,000 x g and 4 ˚C for 10 min to remove residual bacteria.
- Transfer the supernatant to a new microcentrifuge tube without disturbing the bacterial pellet. Use a 1:100 dilution to measure the absorption at 269 nm and 320 nm. The total number of phages can be calculated using the following formula23:
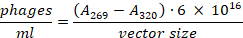
- Store phage library at 4 ˚C for short-term use or with 25% glycerol at -80 ˚C for long-term storage.
- Repeat rounds of selection (steps 3.1–3.6) for 3–6 rounds or until desired enrichment is observed (refer to Results section). Plate and pick single clones (section 4) in order to characterize their affinity and specificity to the ligand (sections 5–7).
4. Single clone isolation
- To isolate individual clones from an enriched sublibrary, prepare 10x serial dilutions of the phage-infected TG1 cells (step 3.5.2). Plate serial dilutions on 90 mm 2YT-agar dishes containing 100 μg/mL ampicillin and 2% (wt/vol) glucose and incubate at 37 ˚C overnight.
- From the overnight plates, pick single colonies into 250 μL of 2YT media supplemented with 100 μg/mL ampicillin per well in sterile deep-well plates and grow at 37 ˚C overnight.
- From the overnight cultures, inoculate 10 µL into 500 µL of fresh 2YT media supplemented with 100 μg/mL ampicillin.
- Grow cells to an OD600 of ~0.5, add CM13 helper phage at 5 x 109 pfu/mL and incubate at 37 ˚C and 250 rpm for 45 min.
- Add 500 µL of 2YT media supplemented with 100 μg/mL ampicillin and 50 μg/mL kanamycin. Incubate at 25 ˚C and 250 rpm overnight.
- Centrifuge the deep-well plates from the overnight cultures at 3,000 x g for 10 min. Collect the supernatant containing the phage particles without disturbing the cell pellet.
- Phage particles can be used for ELISA to determine the specificity of the selected clones to the ligand. Biotin or a structural homolog of the target can be used as a negative control.
5. Anchor binder validation by ELISA
- Coat 96 well ELISA plates using 100 μL of 5 μg/mL streptavidin in coating buffer (100 mM carbonate buffer, pH = 8.6) at 4 ˚C overnight.
- Wash the ELISA plates 3x using 0.05% PBST and add 100 μL of 1 μM biotinylated target to the target wells. Add 100 μL of 1 μM biotin or target homolog to the control wells. Incubate at RT for 1 h.
- Wash plates 5x using 0.05% PBST and block nonspecific binding by adding 300 µL of 1% casein in 1 x PBS. Incubate at RT for 1 h.
- Wash the ELISA plates 3x using 0.05%-PBST and add the purified phage supernatant. Incubate for 1 h at RT.
- Wash the ELISA plates 10x using 0.05% PBST and add 100 μL horseradish peroxidase (HRP)-M13 major coat protein antibody (1:10,000 dilution with 1 x PBS with 1% casein). Incubate at RT for 1 h.
- Wash the ELISA plates 3x using 0.05% PBST and add 100 μL tetramethylbenzidine (TMB) substrate. Incubate for 10 min or until a visible color change is observed. Stop the reaction by adding 100 μL of 1 M HCl. Read the plate at 450 nm on a spectrophotometer.
- For protein expression and purification, choose the clones showing high affinity and specificity for the target (see Discussion).
6. Protein expression, purification, and biotinylation
- As previously reported19, subclone selected clones from section 5 and express as C-terminal Avi-tagged and His-tagged nanobodies.
- Express selected nanobodies in the periplasm of E. coli WK6 cells (typically in 1 L culture), release by osmotic shock, and purify using a nickel-NTA column (see Table of Materials).
- Exchange buffer with a desalting column (1 x PBS with 5% glycerol; see Table of Materials).
- Biotinylate nanobodies using a commercial kit (see Table of Materials) for further use.
7. Anchor binder characterization by BLI
- Analyze the binding affinity and kinetics of selected anchor binders by immobilizing 200 nM biotinylated anchor binders on streptavidin biosensors (see Table of Materials) with binding assay buffer (1 x PBS (pH = 7.4), 0.05% Tween 20, 0.2% BSA, 3% methanol).
- Calculate dissociation constants (KD) of anchor binder-ligand interactions by steady-state analysis using data analysis software (see Table of Materials). Obtained KD values typically range from single- to double-digit micromolar.
8. Dimerization binder screening
NOTE: The biopanning screening of "dimerization binders" is similar to that of anchor binders, except for two critical steps: 1) Dimerization binders are selected using a selected biotinylated anchor binder and the anchor binder-ligand complex for the negative and positive selections, respectively. 2) During the elution step, 100 mM triethylamine is used to elute positively selected phages that were only bound to the anchor binder--ligand target complex. The 100 mM trimethylamine solution (pH = 11.5) is used to elute positive clones by disrupting the protein interactions.
- Beginning of selection
- Begin every round of selection by inoculating a single TG1 cell colony, freshly grown on a minimal media, in 6 mL 2YT at 37 ˚C and 250 rpm to an OD600 of ~0.5. Incubate cells on ice.
- Removal of negatively selected nanobodies
- Prepare the "subtraction tube" by using 400 µL of streptavidin-coated magnetic beads and follow step 3.2. However, instead of saturating with biotin, add 5x the calculated full binding capacity using the selected biotinylated anchor binder and save the unbound phages to be used in step 8.3.3.
- Selection of positively selected nanobodies
- Prepare the "capturing tube" by using 1/2 the volume of streptavidin-coated magnetic beads used for the "subtraction tube" and following steps 3.3.2 to 3.3.3. However, instead of saturating with the biotinylated ligand, add five times the calculated full binding capacity using the selected biotinylated anchor binder.
- To form the anchor binder-ligand complex for the positive dimerization binder selection, add a high enough concentration of non-biotinylated ligand. This will allow most streptavidin-bound anchor binder to form the ligand-bound complex.
- Follow steps 3.3.3 to 3.3.8, using the unbound phages taken from the "subtraction tube".
- Elution of positively selected nanobodies
- Elute the phages bound to the anchor binder-ligand complex by adding 450 µL of 100 mM triethylamine, and incubating at RT on a rotator for 10 min.
- Collect the competitively eluted phages and follow steps 3.4.1 to 3.4.2.
- Further rounds of dimerization binder selection
- Follow steps 3.5 and 3.6 to amplify and recover the library in order to perform further rounds of selection. Repeat rounds of selection for 3–6 rounds or until desired enrichment is observed. Plate and pick single clones (refer to section 4) in order to characterize their affinity and specificity to the target.
9. Dimerization binder characterization by ELISA
- Follow the steps in section 4 to isolate individual clones for characterization via ELISA.
- To test the affinity of dimerization binder candidates to the anchor binder-ligand complex, coat the ELISA target plate using 100 μL of 100 nM biotinylated anchor binder. After incubation for 1 h, add 1 μM of the ligand target to form the anchor binder-ligand complex.
- The control plate should be coated using the biotinylated anchor binder alone to screen out clones that can also bind to the free anchor binder. Add 100 μL of 100 nM biotinylated anchor binder and incubate at RT for 1 h.
- Follow sections 5.3–5.7.
10. Dimerization binder characterization by BLI
- The binding affinity and kinetics of dimerization binders for the anchor binder--ligand complex can be analyzed by immobilizing biotinylated dimerization binders on streptavidin (SA) biosensors with the binding assay buffer and then assayed with 1 μM anchor binder pre-equilibrated with serial dilutions of the ligand. The KD, kon, and koff of the interactions can be calculated using our reported method19.
Access restricted. Please log in or start a trial to view this content.
Results
We describe the two-step in vitro selection and validation of anchor and dimerization binders by screening the combinatorial nanobody library with a diversity higher than 109 using CBD as a target. Assessing the enrichment of the phage biopanning during the successive rounds of selection for both anchor and dimerization binders is important. Typical enrichment results after 4–6 rounds of selection as shown in Figure 5 are a good indication that there is a high ratio of poten...
Access restricted. Please log in or start a trial to view this content.
Discussion
It is critical to choose the correct concentrations of input phage libraries for different rounds of biopanning. We typically started from an input library of ~1012–1013 phage particles with a diversity >109, allowing ~100–1,000 copies of each phage clone to be presented in the pull-down assay. If the phage concentration in a binding assay is too high or low, the likelihood of nonspecific binding or loss of positive clones will increase. The anchor or dimerization binder s...
Access restricted. Please log in or start a trial to view this content.
Disclosures
A provisional patent related to this work has been filed by the University of Washington.
Acknowledgements
This work was supported by the University of Washington Innovation Award (to L.G.), a grant from the U.S. National Institutes of Health (1R35GM128918 to L.G.), and a startup fund of the University of Washington (to L.G.). H.J. was supported by a Washington Research Foundation undergraduate fellowship. K.W. was supported by an undergraduate fellowship from the University of Washington Institute for Protein Design.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| 1-Step Ultra TMB ELISA substrate solution | Thermo Fisher Scientific | 34029 | |
| Agar | Thermo Fisher Scientific | BP1423-2 | |
| Amicon Ultra-15 Centrifugal Filter unit (3 kDa cutoff) | Millipore | UFC900324 | |
| Ampicillin | Thermo Fisher Scientific | BP1760-25 | |
| Bio-Rad Protein Assay Kit II | Bio-Rad | 5000002 | |
| BirA biotin-protein ligase standard reaction kit | Avidity | BirA500 | |
| Bovine Serum Albumin (BSA) | Sigma-Aldrich | A2153-50G | |
| Casein | Sigma-Aldrich | C7078-1KG | |
| CM13 Helper phage | Antibody Design Labs | PH020L | |
| D-(+)-Glucose monohydrate | Alfa Aesar | A11090 | |
| Dynabeads M-280 Streptavidin | Thermo Fisher Scientific | 11205D | |
| DynaMag-2 Magnet | Thermo Fisher Scientific | 12321D | |
| EDTA | Thermo Fisher Scientific | BP120-1 | |
| Fast DNA Ladder | New England Biolabs | N3238S | |
| FastDigest BglI | Thermo Fisher Scientific | FD0074 | |
| Glycerol | Thermo Fisher Scientific | BP229-1 | |
| HiLoad 16/600 Superdex 200 pg | GE Healthcare | 28989335 | |
| HiPrep 26/10 Desalting Column | GE Healthcare | 17508701 | |
| HisTrap-FF-1ml | GE Healthcare | 11000458 | |
| Imidazole | Alfa Aesar | 161-0718 | |
| IPTG | Thermo Fisher Scientific | 34060 | |
| Kanamycin | Thermo Fisher Scientific | BP906-5 | |
| M13 Major Coat Protein Antibody | Santa Cruz Biotechnology | sc-53004 | |
| NaCl | Sigma-Aldrich | S3014-500G | |
| NanoDrop 2000/2000c Spectrophotometers | Thermo Fisher Scientific | ND-2000 | |
| Nunc 96-Well Polypropylene DeepWell Storage Plates | Thermo Fisher Scientific | 260251 | |
| Nunc MaxiSorp | Thermo Fisher Scientific | 44-2404-21 | |
| Octet RED96 | ForteBio | N/A | |
| pADL-23c Phagemid Vector | Antibody Design Labs | PD0111 | |
| PEG-6000 | Sigma-Aldrich | 81260-1KG | |
| Platinum SuperFi DNA Polymerase | Invitrogen | 12351010 | |
| PureLink PCR Purification Kit | Thermo Fisher Scientific | K310001 | |
| QIAprep Spin M13 Kit | Qiagen | 27704 | |
| Recovery Medium | Lucigen | 80026-1 | |
| SpectraMax Plus 384 | Molecular Devices | N/A | |
| Sucrose | Sigma-Aldrich | S0389-1KG | |
| Super Streptavidin (SSA) Biosensors | ForteBio | 18-5057 | |
| Superdex 75 increase 10/300 GL Column | GE Healthcare | 28-9909-44 | |
| T4 DNA Ligase | Thermo Fisher Scientific | 15224-025 | |
| TG1 Electrocompetent Cells | Lucigen | 60502-1 | |
| Triethylamine | Sigma-Aldrich | 471283-100mL | |
| Trizma Base | Sigma-Aldrich | T1503 | |
| Tryptone | Thermo Fisher Scientific | BP9726-5 | |
| Tween 20 | Thermo Fisher Scientific | BP337-500 | |
| Yeast Extract | Thermo Fisher Scientific | BP1422-2 | |
| Zeba Spin Desalting Column | Thermo Fisher Scientific | 89882 |
References
- Stanton, B. Z., Chory, E. J., Crabtree, G. R. Chemically induced proximity in biology and medicine. Science. 359 (6380), (2018).
- Wu, C. Y., Roybal, K. T., Puchner, E. M., Onuffer, J., Lim, W. A. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science. 350 (6258), (2015).
- Straathof, K. C., et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 105 (11), 4247-4254 (2005).
- Di Stasi, A., et al. Inducible apoptosis as a safety switch for adoptive cell therapy. The New England Journal of Medicine. 365 (18), 1673-1683 (2011).
- Mank, M., et al. A FRET-based calcium biosensor with fast signal kinetics and high fluorescence change. Biophysical Journal. 90 (5), 1790-1796 (2006).
- Nagai, T., Sawano, A., Park, E. S., Miyawaki, A. Circularly permuted green fluorescent proteins engineered to sense Ca2+. Proceedings of the National Academy of Sciences of the United States of America. 98 (6), 3197-3202 (2001).
- Hunter, M. M., Margolies, M. N., Ju, A., Haber, E. High-affinity monoclonal antibodies to the cardiac glycoside, digoxin. Journal of Immunology. 129 (3), 1165-1172 (1982).
- Bradbury, A. R. M., Sidhu, S., Dubel, S., McCafferty, J. Beyond natural antibodies: the power of in vitro display technologies. Nature Biotechnology. 29 (3), 245-254 (2011).
- Chen, G., et al. Isolation of high-affinity ligand-binding proteins by periplasmic expression with cytometric screening (PECS). Nature. Biotechnology. 19 (6), 537-542 (2001).
- Tinberg, C. E., et al. Computational design of ligand-binding proteins with high affinity and selectivity. Nature. 501 (7466), 212-216 (2013).
- Spencer, D. M., Wandless, T. J., Schreiber, S. L., Crabtree, G. R. Controlling signal transduction with synthetic ligands. Science. 262 (5136), 1019-1024 (1993).
- Ho, S. N., Biggar, S. R., Spencer, D. M., Schreiber, S. L., Crabtree, G. R. Dimeric ligands define a role for transcriptional activation domains in reinitiation. Nature. 382 (6594), 822-826 (1996).
- Belshaw, P. J., Ho, S. N., Crabtree, G. R., Schreiber, S. L. Controlling protein association and subcellular localization with a synthetic ligand that induces heterodimerization of proteins. Proceedings of the National Academy of Sciences of the United States of America. 93 (10), 4604-4607 (1996).
- Farrar, M. A., AlberolaIla, J., Perlmutter, R. M. Activation of the Raf-1 kinase cascade by coumermycin-induced dimerization. Nature. 383 (6596), 178-181 (1996).
- Erhart, D., et al. Chemical Development of Intracellular Protein Heterodimerizers. Chemistry & Biology. 20 (4), 549-557 (2013).
- Ballister, E. R., Aonbangkhen, C., Mayo, A. M., Lampson, M. A., Chenoweth, D. M. Localized light-induced protein dimerization in living cells using a photocaged dimerizer. Nature Communications. 17 (5), 5475(2014).
- Hill, Z. B., Martinko, A. J., Nguyen, D. P., Wells, J. A. Human antibody-based chemically induced dimerizers for cell therapeutic applications. Nature Chemical Biology. 14 (2), 112-117 (2018).
- Foight, G. W., et al. Multi-input chemical control of protein dimerization for programming graded cellular responses. Nature Biotechnology. 37 (10), 1209-1216 (2019).
- Kang, S., et al. COMBINES-CID: An efficient method for de novo engineering of highly specific chemically induced protein dimerization systems. Journal of the American Chemical Society. 141 (28), 10948-10952 (2019).
- Muyldermans, S. Nanobodies: natural single-domain antibodies. Annual Review of Biochemistry. 82, 775-797 (2013).
- Fanning, S. W., Horn, J. R. An anti-hapten camelid antibody reveals a cryptic binding site with significant energetic contributions from a nonhypervariable loop. Protein Science. 20 (7), 1196-1207 (2011).
- Zavrtanik, U., Luken, J., Loris, R., Lah, J., Hadzi, S. Structural basis of epitope recognition by heavy-chain camelid antibodies. Journal of Molecular Biology. 430 (21), 4369-4386 (2018).
- Denhardt, D. T., Dressler, D., Ray, D. S. The Single-Stranded DNA Phages. , 605-625 (1978).
- Virnekas, B., et al. Trinucleotide phosphoramidites: ideal reagents for the synthesis of mixed oligonucleotides for random mutagenesis. Nucleic Acids Research. 22 (25), 5600-5607 (1994).
- Gu, L., et al. Multiplex single-molecule interaction profiling of DNA-barcoded proteins. Nature. 515 (7528), 554-557 (2014).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved